Open Access Article
Open Access ArticleChloride transport in conductive polymer films for an n-type thermoelectric platform†
Byeonggwan
Kim
 ,
Jong Un
Hwang
and
Eunkyoung
Kim
,
Jong Un
Hwang
and
Eunkyoung
Kim
 *
*
Department of Chemical and Biomolecular Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, South Korea. E-mail: eunkim@yonsei.ac.kr
First published on 8th November 2019
Abstract
Cl− transport in a conductive polymer (CP) film was demonstrated for n-type thermoelectric (TE) harvesting. CPs have been considered as an important group of p-type TE materials due to their high TE functionalities plus simple processing steps for a device. In particular, recently emerging p-type ionic CPs can be unique candidates due to their high Seebeck coefficients (S). However, n-type materials based on CPs suffer from very poor TE functionalities, and n-type ionic TE CP materials have not been realized so far. Here, we report the first example of n-type mixed ionic–electronic CP composite (NPC) films. The p-type TE properties of the PEDOT:PSS films was drastically converted into the n-type TE properties in the presence of CuCl2 through metal binding with polymers, thus resulting in the formation of Cl− channels. Fluorescence imaging using Cl− as an indicator and time-of-flight secondary ion mass spectrometry mapping confirmed that Cl− is transported in the film from the hot to the cold electrode. In addition, electron spin resonance spectroscopy indicated the major spin density transition from a polaron of PEDOT:PSS to the polymer-bound unpaired electron spin of Cu ions by increasing the CuCl2 content to prove the binding of metal ions with the PSS unit of the polymer chain. These mixed ionic–electronic NPC films recorded a surprisingly high negative S value of over −18.2 mV K−1 and a power factor of 1.7 mW m−1 K−2 at 80% RH with 40 wt% of CuCl2. Taking advantage of this high performance, the CP films were integrated with a p-type CP film as a flexible module-type TE harvester with 10 pairs of p–n legs on CNT electrodes. This TE harvester showed a thermovoltage of 1.55 V for a low temperature gradient of 4.5 K. This high anion transport in a TE CP hydrogel film might be a useful solution for environmentally benign and body-worn electronics.
Broader contextThermoelectrics harvest electrical energy directly from waste heat through electron and ion transport between the temperature gradient. While TE materials have been explored to provide increased TE output by optimizing molecular and nanostructures, the output from the currently available TE materials is still low because of the difficulties in controlling the carrier transport. In particular, a high TE performance for flexible and low-cost polymeric TE materials is a challenging issue in wearable thermoelectrics. In this work, we tackled this challenge by optimizing the transport of chloride ions as n-type carriers in PEDOT:PSS films, which further benefited from the formation of copper ion-complexed polymer channels. With such a new n-type TE mechanism, the p-type TE property of the PEDOT:PSS film was drastically converted into an n-type TE property. The Seebeck coefficient and power factor of the n-type film were over −18 mV K−1 and 1.7 mW m−1 K−2, respectively, which provided flexible module-type thermoelectrics, generating 1.55 V for a small ΔT value of 4.5 K. This high anion transport in a polymer film can be useful to power wearable electronics and inspire new strategies for carrier control for mixed ionic–electronic devices. |
Introduction
The importance of Cl− transport has been demonstrated extensively not only for human diseases that are due to mutations in chloride channels but also in the chemical industry for electrochemical devices, anion exchange membranes, anti-corrosion efforts, and drinking water.1 The ions flow through the transporter driven by the concentration gradient of the ions or by imposed stimuli. In particular, under a temperature gradient (ΔT), ion transport generates thermoelectric (TE) output, which has emerged as a promising TE mechanism for self-powered bionics, sensors, and renewable energy harvesters.2–7To this end, eco-friendly and low-cost conductive polymers (CPs) have been explored as unique candidates for p-type TE materials due to their high Seebeck coefficients (S).8,9 In particular, ionic TE materials based on poly(3,4-ethylenedioxythiophene) (PEDOT) doped with poly(styrene sulfonate) (PSS) were explored for high ionic Seebeck coefficients (Si) originating from the Soret effect.10–15 Recently, we demonstrated robust p-type proton-conducting polymer films by using high-molecular-weight poly(styrene sulfonic acid) (PSSH) as a dopant.9 The highly doped PEDOT:PSS containing 30 wt% PSSH (HDH30) recorded high Si (16.2 mV K−1) and power factor (PF, 7.6 mW m−1 K−2). Furthermore, the introduction of self-humidifying layers including a metal–organic framework (MOF-801) and a hydrogel layer on the HDH30 films afforded an environmentally sustainable TE prototype.
To realize an efficient TE power generator and create high-density organic TE legs, a module-type TE harvester should be made using p- and n-type TE materials that are sandwiched between two electrical contacts.16 It is important to understand that in ionic thermoelectric materials, the term n-type (or p-type) is used to describe negative (or positive) ions that can dominantly transport through ΔT. While many examples are reported for n-type inorganic TE materials, it is difficult to find organic or polymeric n-type TE materials with PF comparable to those of inorganic TE materials. An n-type material based on CPs suffers from very poor TE functionalities, and n-type ionic TE CP materials having high PF (>1 mW m−1 K−2) have not been realized so far. Various types of hybrid composites made of organic and inorganic TE materials have been reported since the metal coordination n-type polymer poly[Kx(Ni-ett)] having PF of 66 μW m−1 K−2 was reported.17 Some examples include hybrids of single-walled carbon nanotubes (CNTs) and PEDOT:FeCl4 treated by tetrakis(dimethylamino)ethylene (PF of ∼1050 μW m−1 K−2),18 poly(Ni-ett) film (PF of ∼360 μW m−1 K−2),19 benzyl viologen-doped CNT webs (PF of 3.1 mW m−1 K−2),20 and the hybrid superlattices of TiS2/[(hexylammonium)x(H2O)y(DMSO)] (PF of 450 μW m−1 K−2).21 However, few of them are stable under ambient conditions,22 limiting their practical application. In addition, most of the conductive inorganic or carbon materials have high thermal conductivity (κ),20 which results in a low TE figure of merit, ZT = PF × T/κ, where T is the absolute temperature of the material. Therefore, in order to realize an efficient TE generator, n-type ionic TE materials could be an alternative.
Recently, an anion-doped conjugated polymeric system was introduced utilizing strong electronic interactions between lone-pair electrons of anions (such as F−, OH−, AcO−, Br−, and I−) and π*-orbitals of the π-electron-deficient units (n–π*-interaction), resulting in effective n-doping.23,24 Thus, tetrabutylammonium fluoride (TBAF) was employed as the n-dopant for chlorinated benzodifurandione-based poly(p-phenylene vinylene) (ClBDPPV) to increase σ up to 0.62 S cm−1, with an S of −99.2 ± 9.2 μV K−1 in air.25 The results indicate that small anionic salts such as TBAF as n-dopants of conjugated polymers can provide a strategy toward air-stable n-type CPs. However, the PF of the anion-doped TE materials is still very low because the conductivity is low.
To overcome these obstacles, we hypothesized a free anion-transporting CP film in which anion transport channels are formed by binding the counter cations with mixed ionic–electronic polymers, instead of such strong n–π*-interaction between anions and CPs as discussed above. Moreover, a mixed ionic–electronic system could further enhance TE performance. It has been reported that Cu2+ binds to PSS in the PEDOT:PSS film and increases the conductivity of the film when doped with Cu2+.26 Thus, in this system, the counter anion channel could be formed and then anions may be easily transported to a cold side as ΔT develops. In particular, among the anions, Cl− has one of the highest diffusion coefficients of 2.032 × 10−5 cm2 s−1 in aqueous solution.27 Therefore, the addition of CuCl2 may result in increases in S and σi, leading to increased output voltage (Vout) and output power under small ΔT conditions. With these hypotheses, we embarked on the development of a mixed anionic–electronic TE system.
Herein, we report the first example of n-type mixed ionic–electronic CP films containing CuCl2. The p-type nature of the PEDOT:PSS films was drastically converted into the n-type nature in the presence of CuCl2. Fluorescence imaging and time-of-flight secondary ion mass spectrometry (ToF-SIMS) mapping confirmed that Cl− was transported to the cold side of the film. In addition, electron spin resonance (ESR) spectroscopy indicated a transition from a polaron of the PEDOT:PSS to a free radical of Cu by increasing CuCl2, to prove the binding of metal ions with the PSS unit of the polymer chain. These mixed ionic–electronic NPC films recorded a surprisingly high negative S of over −18.2 mV K−1 and a PF of 1.7 mW m−1 K−2 at 80% RH with 40 wt% of CuCl2. A flexible module-type TE harvester with 10 pairs of p–n legs was fabricated to afford 1.55 V of thermovoltage for a low ΔT of 4.5 K. This high anion transport in a TE CP hydrogel film might be a useful solution for environmentally benign and body-worn electronics.
Results and discussion
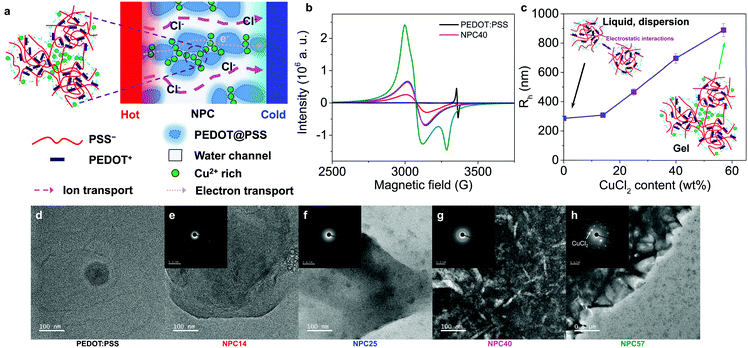
A schematic diagram of the n-type mixed ionic–electronic TE polymer system is shown in Fig. 1a, in which CPs are doped with metal cations (M+), to generate anionic channels. The highly doped PEDOT:PSS (HD) was chosen as a mixed ionic–electronic polymer2,9 and CuCl2 as a binding metal plus anion source. To a solution of PEDOT:PSS, anhydrous CuCl2 was added in concentrations of 14, 25, 36, 40, and 57 wt% (solids content) and dried to afford metal-complexed anionic polymer films, which were named NPC14, NPC25, NPC36, NPC40, and NPC57, respectively.ESR measurements were carried out to study the CuCl2 effect on the nature of the charge carriers in the NPC films. Fig. 1b shows ESR spectra of HD and NPCs (CuCl2 doped HD). For PEDOT:PSS without CuCl2 the peak at 3357 G with g = 2.0011 can be accounted for by the polarons in the PEDOT:PSS film.28 The integrated ESR intensity for PEDOT:PSS decreased by 83% following addition of 14 wt% CuCl2 (NPC14) and there appeared a new broad ESR line at 3078 G with a g = 2.1825. Since the g-factor is lower than 2.3,29 the line could be attributable to the coupling of the unpaired electron spin with PSS. This line became stronger and sharper with increasing CuCl2 content (Fig. S1, ESI†). In the presence of excess CuCl2 (NPC57), another asymmetric peak was observed at 3285 G, which may be due to the presence of the anisotropic film and Cu isotope. Interestingly, the g-factors in the NPC14 to NPC40 were almost the same (2.1825–2.1834) and increased slightly in NPC57 (g = 2.1960). This indicates that the nature of the spin in the Cu ions that bind to the PSS could be the same.
Fig. S2 (ESI†) shows the absorption spectra of NPC films, which show a high bipolaronic absorption of PEDOT at 1610 nm, along with a polaronic band with a maximum at 810 nm. The absorption of the NPC films in the NIR region increased with increasing CuCl2 content. Interestingly, the absorbance intensity (Aλ) in the visible region increased linearly as the CuCl2 content increased. This indicates that CuCl2 binding to PSS led to partial de-doping in PEDOT. The conductivity of the PEDOT:PSS was increased with increasing CuCl2 content, which matches well with a previous report.26
From the X-ray photoelectron spectroscopy (XPS) for NPC films, two Cu 2p peaks30,31 were observed at 932.4 (2p3/2) and 951.9 eV (2p1/2) (Fig. S3a, ESI†). Those two peaks were split into two with a splitting degree of 2.3 eV for both 2p3/2 and 2p1/2, which could be associated with Cu+ and Cu2+, respectively. Additionally, the Cu 2p spectra showed characteristic shake-up satellites peaks. As the Cu2+ concentration increased in the NPC film, the Cu 2p peaks shifted to lower binding energy, and the integration ratio of Cu+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ reached 1
Cu2+ reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.08. This means that a small excess of Cu2+ participated in the binding with polymers in aqueous media. Fig. S3b (ESI†) shows two characteristic peaks of S 2p for PSS− and PEDOT+ at binding energies of 168.2 and 164.0 eV, respectively, for NPC40. Similar to the Cu 2p peaks, the S 2p peak of PSS− shifted from 168.7 to 168.1 eV as the Cu2+ concentration increased because Cu2+ withdraws electron density from PSS−. Based on these results, we conclude that the addition of CuCl2 in PEDOT:PSS produces ionic cross-linking between the PSS shell and Cu2+. This can be beneficial for the generation of a free anion channel in wet conductive polymer media for the n-type ionic TE effect.
1.08. This means that a small excess of Cu2+ participated in the binding with polymers in aqueous media. Fig. S3b (ESI†) shows two characteristic peaks of S 2p for PSS− and PEDOT+ at binding energies of 168.2 and 164.0 eV, respectively, for NPC40. Similar to the Cu 2p peaks, the S 2p peak of PSS− shifted from 168.7 to 168.1 eV as the Cu2+ concentration increased because Cu2+ withdraws electron density from PSS−. Based on these results, we conclude that the addition of CuCl2 in PEDOT:PSS produces ionic cross-linking between the PSS shell and Cu2+. This can be beneficial for the generation of a free anion channel in wet conductive polymer media for the n-type ionic TE effect.
Dynamic light scattering (DLS) measurements of the hydrodynamic radius (Rh) for dilute NPC solutions were plotted against solute weight percent, as shown in Fig. 1c and Fig. S4 (ESI†). We found that the size of the microgels changed upon CuCl2 addition. At 40 and 57 wt%, approximately 14 and 30 dispersed particles aggregated to make a larger particle as a microgel. The zeta potential increased from −29 to −13 mV as the Cu2+ concentration increased from 0 to 57 wt% (Fig. S4c, ESI†). The diffusion of Cu2+ ions to the electrical double layer near the surface of the PEDOT:PSS nanoparticle resulted in an increase in the zeta potential. Additionally, the solution conductivity increased as the Cu2+ concentration increased (Fig. S4d, ESI†). Further identification of the PSS−–Cu2+ was confirmed through 1H NMR for dilute NPC solutions, as described in detail in Fig. S5 (ESI†).
Transmission electron microscopy (TEM, Fig. 1d–h) and scanning electron microscopy (SEM, Fig. S6f–j, ESI†) showed that the nanoparticles of ∼20 nm in size in PEDOT:PSS increased in size to large agglomerates as the Cu2+ concentration increased until it reached 40 wt%. At extremely high concentrations (57 wt%) of CuCl2, large needle-like CuCl2 crystals were found to form an inhomogeneous composition on the NPC57 film. Similarly, the atomic force microscopy (AFM) image of the NPC films under ambient conditions (∼24 °C and ∼50% RH) (Fig. S6m–q, ESI†) showed that the small PEDOT:PSS nanoparticles aggregated to form large agglomerates at a high CuCl2 content. At the same time, the roughness of the film increased as the CuCl2 content increased. This indicated that Cl− channels could be developed between the PEDOT–PSSH–Cu complexes. In the high-resolution X-ray diffraction (HRXRD) patterns, NPC films, except for NPC57, exhibited broad peaks with similar 2θ values at 21° (Fig. S7, ESI†). These results indicate that CuCl2 was well dissolved in NPC media up to 40 wt%, which resulted in poor film quality as seen in NPC57, which exhibited the presence of undissociated CuCl2·2H2O crystalline peaks. Additionally, the morphologies of the PEDOT:PSS and NPC films were stable for 30 days in optical microscopy images except for NPC57 (Fig. S8, ESI†).
As the Cu atom content increased in the films, the sulfur atom content decreased in the EDS images (Fig. S6f–j, ESI†). Interestingly, there were large Cu-rich regions that were segregated by chloride-rich channels, as shown in the EDS for Cu and Cl atoms, respectively. The boundary channels were ∼20 nm wide but, were poorly connected in NPC25; however, they became larger, up to ∼60 nm, and more connected, up to ∼1 μm long, in NPC40. This indicates the formation of chloride channels upon CuCl2 addition.
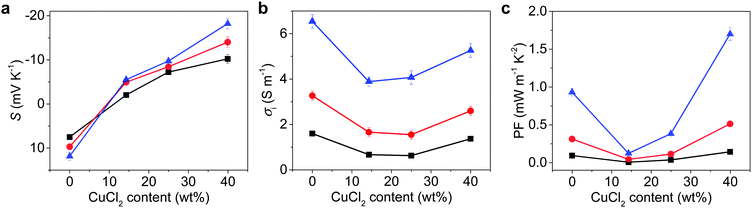
To prove our concept for anion transport for TE media, the thermovoltage (ΔV) was examined at various temperature gradients (ΔT) to obtain the Seebeck constant for NPC films, as detailed in the ESI.† The PEDOT:PSS film showed a high, positive S of 7.5 mV K−1 at 60% RH,9 but the addition of CuCl2 changed it to negative S, proving that the NPCs are n-type (Fig. 2a and Table 1). As the CuCl2 was added to the PEDOT:PSS, S switched signs, changing from 7.5 mV K−1 to −10.2 mV K−1 at 60% RH. The S of NPC14 was −2.0 mV K−1 at 60% RH and showed a higher negative value as the humidity increased, with a value of −5.5 mV K−1 at 80% RH. S was further decreased as the CuCl2 content increased. The highest negative S was found in NPC40 at 80% RH, with a value of −18.2 mV K−1. Interestingly, NPC40 showed a comparably high negative S of −10.2 mV K−1 under ambient conditions (25 °C and 60% RH). An increase in the CuCl2 content up to 46 wt% (NPC46) resulted in a slightly lower S of −17.7 mV K−1 at 80% RH, and it gradually decreased as the CuCl2 content increased. The calculated S values of the NPC films were obtained using the Grotthuss mechanism32 at a uniform gradient of temperature as in eqn (1) (detailed in the ESI†).
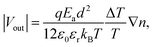 | (1) |
| Sample | Majora carrier, type | RH, % | σ i , S m−1 | σ e , S m−1 | S , mV K−1 | PFe, mW m−1 K−2 | Stability, day | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Major carrier, I for ionic and E for electronic. b Ionic conductivity was determined from the impedance spectroscopy. c Electrical conductivity was determined from the impedance spectroscopy. d Seebeck coefficients in parentheses indicate the Seebeck coefficients after 1 day/30 days stored at the corresponding humidity. e Power factor. Polymer gel: [EMIM][TFSI]/PVDF–HFP polymer gel; CNT–PEDOT composite: sprayed single-walled CNT and PEDOT:FeCl4 treated by tetrakis(dimethylamino)ethylene for 30 min; hybrid superlattice: TiS2/[(hexylammonium)0.08(H2O)0.22(DMSO)0.03] film; doped FBDPPV: FBDPPV doped with 5 wt% N-DMBI; Ag2Se: Ag2Se film on nylon membrane; C60/TiS2: 1 wt% C60/TiS2 nanosheet hybrid film; superlattice STO: superlattice [9 uc SrTiO3/1 un SrTi0.8Nb0.2O3]20[9 uc SrTiO3], 2DESs, MQW where MQW is the multiple quantum-well; uc is the unit cell, 2DES: 2D electron system; superlattice STN/STO: [1 uc SrTi0.4Nb0.6O3|11 uc SrTiO3]10 2DES, ∼5.3 nm thickness; γ-InSe: γ-phase indium selenide, samples with thickness of 7 nm. | ||||||||
| NPC14 | I, n | 60 | 0.67 | 2.7 × 10−3 | −2.0 | 2.7 × 10−3 | >30 | This work |
| 70 | 1.66 | 0.018 | −4.9 | 0.04 | ||||
| 80 | 3.89 | 0.012 | −5.5 | 0.12 | ||||
| NPC25 | I, n | 60 | 0.63 | 0.050 | −7.2 | 0.033 | >30 | |
| 70 | 1.55 | 0.12 | −8.4 | 0.11 | ||||
| 80 | 4.07 | 0.13 | −9.7 | 0.38 | ||||
| NPC36 | I, n | 80 | — | — | −11.2 | — | >30 | |
| NPC40 | I, n | 60 | 1.37 | 0.39 | −10.2 | 0.14 | >30 | |
| 70 | 2.60 | 0.63 | −14 | 0.51 | ||||
| 80 | 5.26 | 0.30 | −18.2 (−18.1/−18.2) | 1.7 | ||||
| NPC57 | I, n | 60 | 26.5 | 0.66 | −8.5 | 1.9 | ∼7 | |
| 70 | 34.0 | 1.08 | −10.3 | 3.6 | ||||
| 80 | 43.0 | 0.83 | −11.2 (−9.5/−5.4) | 5.4 (3.9/1.3) | <1 | |||
| PEDOT:PSS:PSSH | I, p | 80 | 12.2 | 0.03 | 16.0 | 3.1 | >30 | 9 |
| Polymer gel | I, n | — | 0.6 | — | −4 | 9.6 × 10−3 | — | 15 |
| CNT-PEDOT composite | E, n | 35–40 | — | ∼670 | ∼−1.25 | 1.05 | ∼17 | 18 |
| I, n | >90 | ∼5.5 | — | ∼−7.24 | 0.29 | |||
| AgOH-Nafion | I, n | >90 | ∼1 | — | −2 | 4 × 10−3 | 33 | |
| Poly(Ni-ett) film | E, n | — | — | ∼22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
∼−0.126 | ∼0.36 | — | 19 |
| Hybrid superlattice | E, n | — | — | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−0.078 | 0.45 | — | 21 |
| Doped FBDPPV | E, n | — | — | 1400 | −0.174 | 2.8 × 10−3 | — | 34 |
| Ag2Se | E, n | — | — | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
−0.14 | 0.987 | — | 35 |
| C60/TiS2 | E, n | — | — | ∼39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
−0.101 | ∼0.4 | — | 36 |
| Superlattice STO | E, n | — | — | 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−0.85 | 101.15 | 37 | |
| Superlattice STN/STO | E, n | — | — | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
−0.275 | 4.7 | 38 | |
| γ-InSe | E, n | — | — | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
−0.41 | ∼2.3 | 39 | |
A recent study reported the n-type S of −4 ± 0.2 mV K−1 for 1-ethyl-3-methylimidazolium ([EMIM]) bis(trifluoro-methylsulfonyl)imide ([TFSI])/poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF–HFP) polymer gels.15 The S of NPC40 in this work is 4.6 times larger than the reported values of S in the TE films. Such an increase in the S of the NPC films can be attributed to the high σi and the fast Cl− ion mobility in the ion channel being less affected by ionic cross-linked Cu2+ to PSS− (Fig. S4, ESI†). At the optimized CuCl2 content for Cu ion binding with PSS, the free Cl− ions became more mobile through the ion channel, resulting in a higher Soret effect. The Cl− ion-transport number (ti), determined from electrochemical impedance analysis, was over 0.93 at 80% RH for NPC films (Table S1, ESI†).
To identify the contribution from ionic and electronic carriers, electrochemical impedance spectroscopy was performed under an alternating voltage of 0.1 V across the NPC films at various humidity levels (Fig. S9, ESI†). The σi was much larger than σe (Table 1), therefore, the electronic contribution to TE of NPC seemed quite low. The σi increased as the CuCl2 content increased. At 60% RH, the σi of the NPC14 and NPC40 films were 0.67 and 1.37 S m−1, and they increased further as the humidity increased to 3.89 and 5.26 S m−1 at 80% RH, respectively (Fig. 2b). The NPC57 film initially showed a high σi, which may be affected by high instant ion concentration (Cl−) in aqueous media, as observed in the media having high ion concentration.27 Increasing the humidity of the environment is known to enhance the ionic transport in PEDOT:PSS. Since Cl− ions readily dissociate from Cu2+ in PEDOT:PSS, they become highly mobile in humid media and could be influenced by humidity; indeed, the NPC film showed a large dependence of σi and Si on the humidity as well as the content of CuCl2. Thus, TE from NPCs could be mainly attributed to the ion transport process. Because the concentration of chloride is 7.6 times higher (in NPC40) than that of protons from pristine PEDOT:PSS, the major ion here could be Cl−.
The PF of NPC40 was 1.7 mW m−1 K−2 at 80% RH, which is 1.9 and 14.8 times higher than that of p-type PEDOT:PSS and n-type NPC14, respectively, under the same conditions (Fig. 2c). NPC57 initially showed a high PF because of high σi, but CuCl2 in the NPC57 was soon crystallized and led to inhomogeneous film as shown in Fig. 1h. Accordingly, the film showed lower PF (Table 1), as the inhomogeneous CuCl2 crystals may block ion transport. The state-of-the-art PF of polymer-based n-type ionic TE material in the literature was 9.6 μW m−1 K−2, which was observed from a polymer gel.15 Compared to this value, the PF of NPC40 was improved by 177 times. Furthermore, the PF of NPC40 was 1.6 times higher than the highest PF (1050 μW m−1 K−2) of the polymer-based n-type electronic material in the literature, which was obtained from a single-walled CNT and PEDOT:FeCl4 composite treated by tetrakis(dimethylamino)ethylene.18 These results can be attributed to the large increases in Si and σi, which may have originated from the free Cl− and effective Cl− transport through the ion channel in NPC films (Fig. 1a).
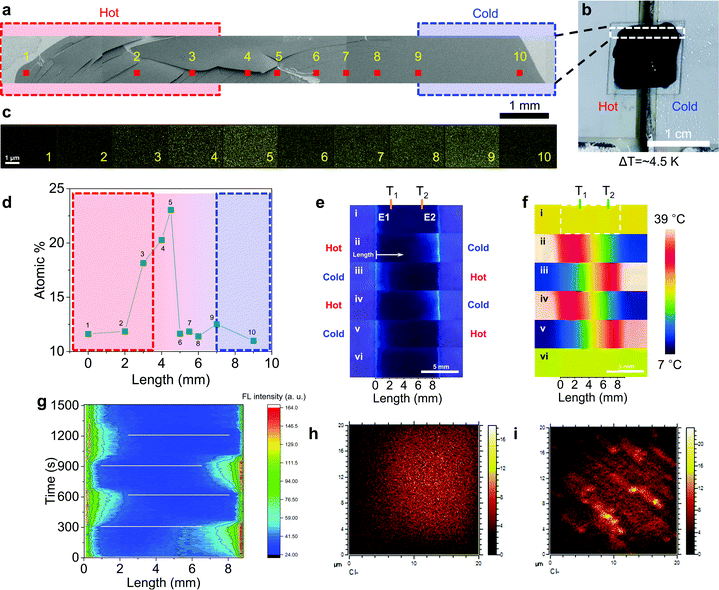
For the direct confirmation of Cl− transport, an NPC40 film on ITO glass was quickly dried under vacuum (∼1 mTorr) when the film was producing Vout of 82 mV at ΔT of 4.5 K and 80% RH for 10 min. The film was further dried in an SEM chamber under high vacuum (∼10−4 Torr) (Fig. 3a and Fig. S10, ESI†). Ten points (area of 4 × 3 μm2) were selected for EDS mapping according to the position of the film on hot and cold Peltier devices (Fig. 3b). The atomic% of Cl at different points were plotted and shown in Fig. 3d. Near the boundary of the hot side and gap area, the atomic% of Cl gradually increased, and it sharply decreased toward the cold side near the center of the film. The maximum atomic% (∼1 mm from the hot edge) was ∼2 times higher than that of normal points. This result shows clear transport of Cl− in the NPC40 film under ΔT to explain the high S and Vout in one film.
Further Cl− transport under ΔT was clearly verified through fluorescence imaging, in which N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE) was used as the Cl− indicator (Fig. S11, ESI†). Initially, without ΔT, the MQAE-stained NPC40 (at 80% RH) showed poor fluorescence contrast as Cl− ions were spread over the film (Fig. 3e, g and Fig. S12, ESI†). Interestingly, the NPC40 showed dramatic fluorescence contrast between the hot and cold electrodes. Thus, when electrode 1 (E1) was heated while the electrode 2 (E2) was left cold, the fluorescence intensity was increased at cold E2 while the hot part remained dark (Fig. 3e-ii). This indicates that Cl− ions were transferred to the cold side. The temperature of each electrode was reversed to determine whether the fluorescence intensity was higher at the cold electrode (Fig. 3e–g). Furthermore, the fluorescence switching was reversible by the temperature switching. The confocal image of the NPC40 film showed similar Cl− transport. Thus, the cold part (bottom) of the NPC film was brighter than the hot part (top) as ΔT (3 K) was applied. This effect can be explained by the Cl− ion transport from the hot part to the cold part. Additionally, when the temperature difference was reversed, the cold part (top) became brighter than the hot part (bottom) over time. Interestingly, the MQAE-stained NPC40 film showed reversible fluorescence switching with repeated heating–cooling cycles (Fig. S13, ESI†). Thermodiffusion of the fluorescent moiety was saturated after ∼1.5 min. Additionally, this dried film was cross-cut and the ToF-SIMS map of the chlorine distribution on the cross-section surface was measured in a negative ion mode (Fig. 3h, i and Fig. S14, ESI†). Bundle-like structures with Cl− clusters were found and the thickness was ∼170 nm. Furthermore, the Cl− intensity at the cold part was 1.8 times larger than the hot part.
The Vout of n-type NPC films was linearly dependent on ΔT (Fig. 4a) and reproducible under three repeated heating–cooling cycles, indicating the high stability of the NPC films (Fig. 4b). Additionally, the generated Vout was well maintained up to 500 s. To determine the output power from NPC40, the film was connected to an external load resistor, and the variations in Vout, current (Isc), and output power of the NPC40 film were measured at a ΔT of 4.5 K and 80% RH. By dividing the cross-sectional area, the output power density of the NPC40 film was maximized to 0.38 mW m−2 by connecting it with a resistance of 300 kΩ from impedance matching (Fig. S15, ESI†). In addition, the specific (Cs) and areal capacitance (Ca) of the NPC40 film on Au and CNT electrodes were estimated from cyclic voltammograms (Fig. S16a, ESI†). The NPC40 film on CNT electrodes showed Cs and Ca of 5.65 F g−1 and 0.22 F cm−2 at 80% RH, respectively, which are 4.6 times higher than those of the NPC40 film on Au electrodes because of the high surface area of the CNT electrode (Fig. S17, ESI†). Additionally, the stability of the NPC40 film on CNT electrodes was investigated as a function of time at 80% RH and 25 °C. The NPC40 film on CNT electrodes was stable for 10 days, maintaining 97.5% of the pristine capacitance from the cyclic voltammograms (Fig. S16b, ESI†).
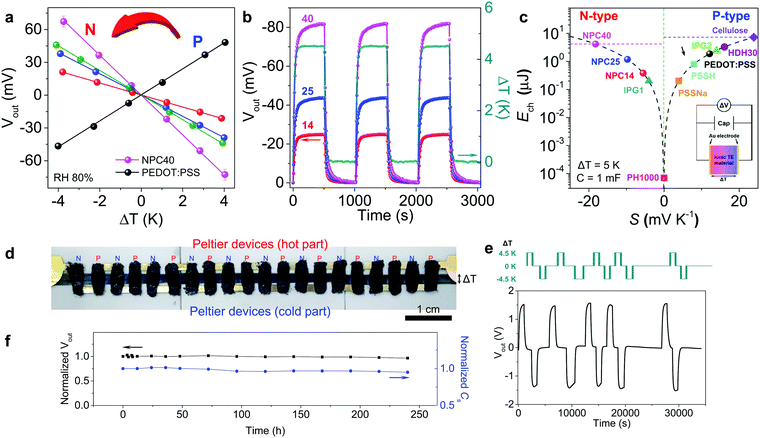 | ||
| Fig. 4 Thermoelectric performance of NPC films and a flexible module-type device. (a) Output voltage at different ΔT values and 80% RH. The slope of the linear fit of the data for the Seebeck coefficient. PEDOT:PSS (black), NPC14 (red), NPC25 (blue), NPC40 (magenta), and NPC57 (green). (b) Output voltage of NPC films under 10 repeated heating–cooling cycles at ΔT of 4.5 K and 80% RH. (c) The charged energy to a capacitor from different thermoelectric materials (ΔT = 5 K, C = 1 mF). Ech of cellulose membrane, PSSNa, PSSH, ionic polymer gel (IPG), and HDH30 were calculated from the S in ref. 8, 9 and 13–15, respectively. Inset: Equivalent circuit for thermal charge–discharge with capacitor. (d) The flexible module-type TE harvester composed of 10 pairs of p–n legs on CNT electrodes. (e) Reproducible output voltage buildup under 10 repeated heating–cooling cycles. (f) Stability testing of the output voltage and specific capacitance for 10 days. The harvester was stored under ambient conditions (25 °C and 50% RH) and measured at a ΔT of 4.5 K and 80% RH. | ||
Fig. 4c shows the current status of electrochemical energy storage (Ech) in different n- and p-type TE capacitors, in which the reported S values were converted into Ech for a ΔT of 5 K and C of 1 mF for comparison (see details in the ESI†). NPC14, NPC25, and NPC40 had Ech values of 0.38, 1.2, and 4.1 μJ at 80% RH, respectively. The thermal conductivity of the NPC40 was determined as 0.26 and 0.34 W m−1 K−1 at 60 and 80% RH, respectively. Considering the above TE properties, ZT and theoretical maximum efficiency were calculated as 1.54 and 0.34% at 298 K, a ΔT of 4.5 K, and 80% RH. These values are much higher than those of other materials in the literature.13–15 Compared with p-type HDH30, the Ech value of NPC40 was 1.3 times higher, which makes it a promising n-type ionic TE material for a module-type flexible TE device.
Taking advantage of the high negative S and stable voltage generation, NPC40 film was integrated with p-type HDH30 film9 as a flexible module-type TE harvester. This module-type TE harvester had 10 pairs of p–n legs (each area of 2 × 4 mm2) on CNT electrodes (Fig. 4d). The Vout for the module-type TE harvester reached 1.55 V at a ΔT of 4.5 K and 80% RH (Fig. 4e). Thus, one pair of p–n legs generated ∼155 mV, with S of 34.4 mV K−1, which is similar to the sum of S for each n-type (18.2 mV K−1) and p-type (16.0 mV K−1) film. This is, to our knowledge, the highest S (n, p) achieved to date in an organic-based TE module. The Vout showed stable and reproducible build-up under 10 repeated heating–cooling cycles. Additionally, it showed stable Vout and Cs values with small losses of 3.5% and 5%, respectively, in a stability test for 10 days (Fig. 4f). Such a large and stable Vout build-up without Vout loss was first demonstrated in the module-type TE harvester based on conductive polymers. This module-type TE platform can be used for compact energy harvesting and body-worn or integrated electronics.
Conclusions
In summary, a high-performance n-type ionic TE film was demonstrated under ambient conditions. The addition of CuCl2 in a PEDOT:PSS solution produced free Cl− anions in the ion channel in wet conductive polymer media. The morphology and homogeneity study of the NPC film elucidated PEDOT-rich and PSS-rich parts and ion channels in the NPC media. The NPC40 film showed a surprisingly high negative S value of −18.2 mV K−1 and a PF of 1.7 mW m−1 K−2 at 80% RH, which, to the best of our knowledge, is the highest among conductive polymers. Additionally, a flexible module-type TE harvester with 10 pairs of p–n legs on CNT electrodes showed 1.55 V of Vout at a ΔT of 4.5 K and 80% RH. One pair of p–n legs had S of 34.4 mV K−1, which is similar to the sum of S for each n-type and p-type legs. From these results, high anion transport in TE materials can be a useful solution for environmentally benign and body-worn electronics.Experimental
Preparation of n-type PEDOT:PSS–CuCl2 (NPC) films
PEDOT:PSS solutions were prepared by filtering with a PTFE filter (ADVANTEC, pore size of 0.45 μm) before use. To obtain various n-type PEDOT:PSS–CuCl2 (NPC) solutions, 14, 25, 40, and 57 wt% CuCl2 (solid content) were added to the PEDOT:PSS solution and stirred for 5 min under an Ar atmosphere to dissolve all solids. Polyethylene terephthalate (PET) substrates (thickness of 0.5 mm) were topped with gold electrodes patterned by thermal evaporation (thickness of 100 nm, width of 1 mm, and distance between electrodes of 3 mm). The metal patterned PET substrate was pretreated with ultraviolet/ozone (UVO) for 5 min to generate a hydrophilic surface. Then, 60 μl aliquots of the solutions were drop-cast onto the above pretreated substrate (area of 0.7 × 0.7 cm2) and dried under ambient conditions (∼24 °C and ∼50% RH) for 6 h or in an Ar atmosphere chamber (∼25 °C) with a flow of 1 sccm Ar for 1.5 h. For the CNT/Au electrode sample, a CNT dispersion in water (0.01 wt% of SWCNTs and 0.1 wt% of DWCNTs) was prepared by ultrasonication for 30 min (750 W, 40%, 2 s pulse). Next, the CNT solution was dropped on a Au electrode (5 μl mm−2) during heating at 60 °C for 1 h to afford a thickness of 10 μm. After water evaporation, the electrodes were rinsed with distilled water before use. For the ionic and electronic measurements, two unsheathed wires were soldered by indium wire to the two metal electrodes using an Ultrasonic Soldering Machine (Mecs Tech Co., Ltd).Fabrication of the TE module
PET substrates (thickness of 0.5 mm) were topped with alternating gold electrodes patterned by thermal evaporation (thickness of 100 nm, width of 2 mm, length of 6 mm, and distance between electrodes of 4 mm). Then, 20 μl of the n- and p-type solutions were drop-casted alternatively onto the pretreated substrate above (area of 2 × 6 mm2) and dried under ambient conditions (∼24 °C and ∼50% RH) for 6 h. The number of legs was 10 for each type of film. The CNT/Au electrode and wiring were achieved by the same method as above.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by a National Research Foundation (NRF) grant funded by Korean government (Ministry of Science, ICT & Future Planning, MSIP) through the Global Research Lab (GRL: 2016K1A1A2912753) and Creative Materials Discovery Program (2018M3D1A1058536).References
- E. Park, E. B. Campbell and R. MacKinnon, Nature, 2016, 541, 500 CrossRef PubMed.
- S. Park, S. W. Heo, W. Lee, D. Inoue, Z. Jiang, K. Yu, H. Jinno, D. Hashizume, M. Sekino, T. Yokota, K. Fukuda, K. Tajima and T. Someya, Nature, 2018, 561, 516–521 CrossRef CAS.
- F. Zhang, Y. Zang, D. Huang, C.-A. Di and D. Zhu, Nat. Commun., 2015, 6, 8356 CrossRef CAS PubMed.
- H. Kim, S. Yang, S. R. Rao, S. Narayanan, E. A. Kapustin, H. Furukawa, A. S. Umans, O. M. Yaghi and E. N. Wang, Science, 2017, 356(6336), 430–434 CrossRef CAS PubMed.
- B. Russ, A. Glaudell, J. J. Urban, M. L. Chabinyc and R. A. Segalman, Nat. Rev. Mater., 2016, 1, 16050 CrossRef CAS.
- L.-D. Zhao, G. Tan, S. Hao, J. He, Y. Pei, H. Chi, H. Wang, S. Gong, H. Xu, V. P. Dravid, C. Uher, G. J. Snyder, C. Wolverton and M. G. Kanatzidis, Science, 2016, 351, 141–144 CrossRef CAS.
- O. Bubnova, Z. U. Khan, A. Malti, S. Braun, M. Fahlman, M. Berggren and X. Crispin, Nat. Mater., 2011, 10, 429–433 CrossRef CAS.
- T. Li, X. Zhang, S. D. Lacey, R. Mi, X. Zhao, F. Jiang, J. Song, Z. Liu, G. Chen, J. Dai, Y. Yao, S. Das, R. Yang, R. M. Briber and L. Hu, Nat. Mater., 2019, 18, 608–613 CrossRef CAS.
- B. Kim, J. Na, H. Lim, Y. Kim, J. Kim and E. Kim, Adv. Funct. Mater., 2019, 29, 1807549 CrossRef.
- P. Qiu, M. T. Agne, Y. Liu, Y. Zhu, H. Chen, T. Mao, J. Yang, W. Zhang, S. M. Haile, W. G. Zeier, J. Janek, C. Uher, X. Shi, L. Chen and G. J. Snyder, Nat. Commun., 2018, 9, 2910 CrossRef.
- H. Wang, U. Ail, R. Gabrielsson, M. Berggren and X. Crispin, Adv. Energy Mater., 2015, 5, 1500044 CrossRef.
- D. Zhao, S. Fabiano, M. Berggren and X. Crispin, Nat. Commun., 2017, 8, 14214 CrossRef CAS.
- H. Wang, D. Zhao, Z. U. Khan, S. Puzinas, M. P. Jonsson, M. Berggren and X. Crispin, Adv. Electron. Mater., 2017, 3, 1700013 CrossRef.
- S. L. Kim, H. T. Lin and C. Yu, Adv. Energy Mater., 2016, 6, 1600546 CrossRef.
- D. Zhao, A. Martinelli, A. Willfahrt, T. Fischer, D. Bernin, Z. U. Khan, M. Shahi, J. Brill, M. P. Jonsson, S. Fabiano and X. Crispin, Nat. Commun., 2019, 10, 1093 CrossRef.
- Q. Zhang, J. Liao, Y. Tang, M. Gu, C. Ming, P. Qiu, S. Bai, X. Shi, C. Uher and L. Chen, Energy Environ. Sci., 2017, 10, 956–963 RSC.
- Y. Sun, P. Sheng, C. Di, F. Jiao, W. Xu, D. Qiu and D. Zhu, Adv. Mater., 2012, 24, 932–937 CrossRef CAS.
- H. Wang, J.-H. Hsu, S.-I. Yi, S. L. Kim, K. Choi, G. Yang and C. Yu, Adv. Mater., 2015, 27, 6855–6861 CrossRef CAS.
- Y. Sun, L. Qiu, L. Tang, H. Geng, H. Wang, F. Zhang, D. Huang, W. Xu, P. Yue, Y.-S. Guan, F. Jiao, Y. Sun, D. Tang, C.-A. Di, Y. Yi and D. Zhu, Adv. Mater., 2016, 28, 3351–3358 CrossRef CAS.
- C. J. An, Y. H. Kang, H. Song, Y. Jeong and S. Y. Cho, J. Mater. Chem. A, 2017, 5, 15631–15639 RSC.
- C. Wan, X. Gu, F. Dang, T. Itoh, Y. Wang, H. Sasaki, M. Kondo, K. Koga, K. Yabuki, G. J. Snyder, R. Yang and K. Koumoto, Nat. Mater., 2015, 14, 622 CrossRef CAS.
- S. Wang, H. Sun, U. Ail, M. Vagin, P. O. Å. Persson, J. W. Andreasen, W. Thiel, M. Berggren, X. Crispin, D. Fazzi and S. Fabiano, Adv. Mater., 2016, 28, 10764–10771 CrossRef CAS.
- R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533 CrossRef CAS.
- S. Guha, F. S. Goodson, L. J. Corson and S. Saha, J. Am. Chem. Soc., 2012, 134, 13679–13691 CrossRef CAS PubMed.
- X. Zhao, D. Madan, Y. Cheng, J. Zhou, H. Li, S. M. Thon, A. E. Bragg, M. E. DeCoster, P. E. Hopkins and H. E. Katz, Adv. Mater., 2017, 29, 1606928 CrossRef.
- J. Ouyang, Displays, 2013, 34, 423–436 CrossRef CAS.
- P. Vanysek and D. R. Lide, in CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, Boca Raton, FL, 85th edn, 2005, pp. 5–94 Search PubMed.
- J. Ouyang, Q. Xu, C.-W. Chu, Y. Yang, G. Li and J. Shinar, Polymer, 2004, 45, 8443–8450 CrossRef CAS.
- H. Xue and S. Schlick, Macromolecules, 1992, 25, 4437–4441 CrossRef CAS.
- C. S. Fadley, Surf. Interface Anal., 2008, 40, 1579–1605 CrossRef CAS.
- M. Xue and Y. Tan, Nanoscale, 2014, 6, 12500–12514 RSC.
- M. A. Reznikov, MRS Proc., 2011, 1325, mrss11 Search PubMed.
- W. B. Chang, C. M. Evans, B. C. Popere, B. M. Russ, J. Liu, J. Newman and R. A. Segalman, ACS Macro Lett., 2016, 5, 94–98 CrossRef CAS.
- K. Shi, F. Zhang, C.-A. Di, T.-W. Yan, Y. Zou, X. Zhou, D. Zhu, J.-Y. Wang and J. Pei, J. Am. Chem. Soc., 2015, 137, 6979–6982 CrossRef CAS.
- Y. Ding, Y. Qiu, K. Cai, Q. Yao, S. Chen, L. Chen and J. He, Nat. Commun., 2019, 10, 841 CrossRef.
- L. Wang, Z. Zhang, L. Geng, T. Yuan, Y. Liu, J. Guo, L. Fang, J. Qiu and S. Wang, Energy Environ. Sci., 2018, 11, 1307–1317 RSC.
- H. Ohta, S. Kim, Y. Mune, T. Mizoguchi, K. Nomura, S. Ohta, T. Nomura, Y. Nakanishi, Y. Ikuhara, M. Hirano, H. Hosono and K. Koumoto, Nat. Mater., 2007, 6, 129 CrossRef CAS.
- Y. Zhang, B. Feng, H. Hayashi, C.-P. Chang, Y.-M. Sheu, I. Tanaka, Y. Ikuhara and H. Ohta, Nat. Commun., 2018, 9, 2224 CrossRef.
- J. Zeng, X. He, S.-J. Liang, E. Liu, Y. Sun, C. Pan, Y. Wang, T. Cao, X. Liu, C. Wang, L. Zhang, S. Yan, G. Su, Z. Wang, K. Watanabe, T. Taniguchi, D. J. Singh, L. Zhang and F. Miao, Nano Lett., 2018, 18, 7538–7545 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ee02399b |
| This journal is © The Royal Society of Chemistry 2020 |