Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chelated Fischer carbene complexes of annulated thiophenes: synthesis, structure and electrochemistry†
Zandria
Lamprecht
 a,
Frederick P.
Malan
a,
Frederick P.
Malan
 a,
Israel
Fernández
a,
Israel
Fernández
 b,
Simon
Lotz
a and
Daniela I.
Bezuidenhout
b,
Simon
Lotz
a and
Daniela I.
Bezuidenhout
 *c
*c
aDepartment of Chemistry, University of Pretoria, Private Bag X20, Hatfield 0028, Pretoria, South Africa
bDepartamento de Química Orgánica I and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Facultad de Ciencias Químicas, Universidad Complutense de Madrid, 28040, Madrid, Spain
cLaboratory of Inorganic Chemistry, Environmental and Chemical Engineering, University of Oulu, P. O. Box 3000, 90014 Oulu, Finland. E-mail: daniela.bezuidenhout@oulu.fi
First published on 23rd October 2020
Abstract
Two (thieno[3,2-b]thiophene) and three annulated thiophenes (dithieno[2,3-b;3′,2′-d]thiophene and dithieno[3,2-b;2′,3′-d]thiophene) were employed as building blocks to synthesize linear or semi-circular chelated mononuclear biscarbene and dinuclear tetracarbene complexes. The electronic properties of the annulated thienylene chelated carbene complexes were investigated by cyclic voltammetry experiments and compared to non-chelated Fischer-type monocarbene complexes. Density functional theory (DFT) calculations were used to assign the redox events and to probe the extent of electron delocalisation as well as the possibility of electronic (intramolecular metal–metal) communication as a result of intervalence. The differences of these electronic properties in the conjugated chelated carbene complexes are compared to chelated carbene compounds without a linear conjugated pathway.
Introduction
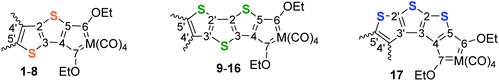
Thiophene oligomers (and polymers) are widely studied because of their electroconductive and photonic properties.1–14 Application of α-connected, conjugated thiophene units in chain stuctures1,5–7 and α,β-fused thiophene rings in band structures1,2,4,8–12 are finding wide application as organic conductors/superconductors4,7,8 and non-linear optical materials.1,5,6,9 In addition, oligothiophenes are appealing units to act as spacer-ligands between metal fragments for potential long distance metal–metal communication.3,13,14 Electron excessive thiophene donors and strong electron-withdrawing Fischer carbene carbon acceptors arranged in a ‘push–pull’ fashion via a conjugated pathway are conducive to electron transfer and polarisation processes with potentially diverse electronic applications.Unlike chelated N-heterocyclic biscarbene complexes, chelated Fischer carbene complexes (FCCs) reported in the literature are more rare.15–31 Dinuclear chelated tetracarbene complexes could be prepared utilising thiophene or thieno[2,3-b]thiophene if the tetrabrominated thienyl substrates were employed as starting materials.25,32 Both chelated mononuclear biscarbene and dinuclear tetracarbene complexes were isolated, as well as the intermediate dinuclear triscarbene complex (with a monocarbene ligand on one metal terminal, and a chelated biscarbene metal carbonyl group on the other side of the spacer thienothiophene with the sulphur atoms both orientated in the same (cis) direction, up-up), Fig. 1. The tetracarbene complex, due to the curved nature of the thienothiophene backbone, displayed extreme steric congestion of the two adjacent ethoxy carbene substituents resulting in an accompanying deviation from planarity.
 | ||
| Fig. 1 Bis-, tris- and tetracarbene complexes reported using tetrabromothieno[2,3-b]thiophene as precursor. | ||
In this study, two fused thiophenes (thieno[3,2-b]thiophene (trans-TT)) and three annulated thiophenes (dithieno[2,3-b;3′,2′-d]thiophene (cis-DTT) and dithieno[3,2-b;2′,3′-d]thiophene (trans-DTT)), are employed as building blocks to synthesize chelated FCCs. For trans-TT and trans-DTT the sulphur atom orientations are in up-down and up-down-up positions, respectively, and expected to give linearly arranged bands of chelated tetracarbene complexes. For comparison, the synthesis and structure of FCCs with the curved cis-DTT isomer with sulphur atoms in an up-up-up orientation, is also investigated. The effect of the conjugation pathway in the different annulated (di)thienothienyl carbene substituents, the number of annulated thienylene rings and the effect of carbene chelation on the electrochemistry of the resultant complexes are investigated.
Results and discussion
Synthesis of chelated carbene complexes
The synthesis of the chelated carbene complexes from thienylene substrates requires activation of both the α- (C5 and C5′) and β-positions (C4 and C4′) of the annulated thiophenes by preparing the tetrabromothienylene derivatives as starting materials.25 The α-positions are generally more reactive compared to the β-positions, allowing some control during a selective step-wise lithiation procedure. The aim of the method in this study is to produce bischelated dimetal tetracarbene complexes, hence the experimental conditions were selected to target these complexes. For annulated thiophene rings this would mean utilizing all available sites for carbene formation. The first dilithiation at C5 and C5′, at a sufficiently low temperature with n-BuLi, leaves C4 and C4′ unaffected. Two equivalents of metal hexacarbonyl are added, permitting nucleophilic attack of the dianions on carbonyl carbon atoms of the metal hexacarbonyls, leading to the formation of a C5,C5′ diacyl-dimetallate. Charge delocalisation in the two substituents, from the metals to oxygens, protects the acyl carbons against further nucleophilic attack. Dilithiation via lithium-halogen exchange of the remaining two sites, C4 and C4′, followed by in situ reaction with two cis carbonyl ligands of the already coordinated metal carbonyl moieties, afford, after alkylation, the dinuclear tetracarbene complex. Alkylations during chelated carbene complex synthesis, are carried out using a large excess of [Et3O][BF4], as extra alkylating agent facilitates the removal of remaining bromines from intermediates.33,34 The reactions are illustrated in Scheme 1. | ||
| Scheme 1 Preparation of new chelated FCCs of group 6 (Cr, W) metal carbonyls from tetrabrominated (di)thienothiophene precursors with sulphur atoms in the up-down(-up) orientation. | ||
In reactions (a) and (b) (Scheme 1), trans-TT-Br4 is used as the starting material, with either chromium (a) or tungsten hexacarbonyl (b) as metal precursors (Scheme 1). The mononuclear chelated biscarbene complexes, [M(CO)4{C(OEt)}2-5,4-C6H2S2] with M = Cr (1, 24% yield) and M = W (5, 26% yield), form through lithiation at only one α-position (C5) of trans-TT-Br4 during the first lithiation step and reaction with a carbonyl ligand of the metal carbonyl precursor. Anion protection via the metal acylate also facilitates a second lithium-halogen exchange reaction on an adjacent β-position. During the second lithiation step, an anion forms at C4 (β-position), converting the acylate into a 5,4-metallacyclic bisacylate. The neutral mononuclear chelated biscarbene complexes are generated after alkylation with excess [Et3O][BF4]. The second mononuclear chelated biscarbene complexes have an additional butyl group attached to the C5′-positions and H/Br on the C4′-positions, [M(CO)4{C(OEt)}2-5,4-C6H0/1S2-5′-C4H9-4′-Br/H] with M = Cr (2, 18% yield) and M = W (6, 15% yield). During the first dilithiation, lithium-halogen exchange on the C5′-position results in the formation of BuBr, followed by electrophilic substitution of BuBr at the C5′-position (lithiated site). The presence of a bromine atom at the C4′-position is an indication of an incomplete second lithiation.
The targeted bischelated dimetal tetracarbene complexes, [{M(CO)4[C(OEt)]2}2-5,4,5′,4′-C6S2] with M = Cr (3, 28% yield) and M = W (8, 15% yield), formed as major products of the reaction. The low yield of 8 is a result of by-product formation, the second modified dimetal carbene complex [W(CO)4{C(OEt)}2-5,4-C6S2-5′-OEt-4′-C{CH2CH3}2C(OEt){W(CO)5}] 7. The mechanism for the formation of 7 is not clear, but Ccarb–Ccarb coupling, bond-breaking and formation reactions are required. However, it is clear that the acylate precursor intermediate for 7 is the same as that required for 8 and modifications could reasonably occur either before or after alkylation. The second dinuclear carbene complex, from the reaction with chromium hexacarbonyl, is the triscarbene complex [Cr(CO)4{C(OEt)}2-5,4-C6HS2-5′-C(OEt)Cr(CO)5] (4, 2% yield). This product is an indication that the second lithiation step resulted in only one acylate to be converted into a 5,4-metallacyclic bisacylate, and the C5′-acylate is left intact.
trans-DTT-Br4 was used as starting material in the next two reactions (Scheme 1(c) and (d)). One mono- and two dimetal carbene complexes form during the reaction with chromium hexacarbonyl and with trans-DTT as spacer. The dimetal complexes are of two types, i.e. a biscarbene and a bischelated tetracarbene complex. Repeating the reaction with tungsten hexacarbonyl, results in two mono- and three dinuclear carbene complexes. The anticipated mononuclear chelated biscarbene complexes, [M(CO)4{C(OEt)}2-5,4-C8H2S3] with M = Cr (9, 3% yield) and M = W (12, 2% yield), formed in trace amounts. Dimetal tetracarbene complexes, [{M(CO)4[C(OEt)]2}2-5,4,5′,4′-C8S3] M = Cr (11, 43% yield) and M = W (16, 51% yield), formed as the targeted major products. The known chromium and tungsten biscarbene complexes of trans-DTT, with C5 and C5′ pentacarbonyl carbene fragments (10 and 13 respectively), formed in trace amounts (<5% yield, Scheme 1(c) and (d)).35 Their formation is a result of dilithiation at the α-positions, along with nucleophilic attack of the dianions on carbonyl carbon atoms of two metal hexacarbonyls, followed by quenching the resulting diacyl-dimetallates with [Et3O][BF4]. For the tungsten reaction (Scheme 1(d)), [W(CO)4{C(OEt)}2-5,4-C8H0/1S3-5′-C4H9-4′-Br/H] (14) and [W(CO)4{C(OEt)}2-5,4-C8H1S3-5′-C(OEt)W(CO)5] (15) were additionally isolated in low yields.
The chelated carbene synthesis reactions, carried out with cis-DTT-Br4 as starting material (Scheme 2), did not proceed smoothly. The use of chromium hexacarbonyl as metal precursor yielded no stable, isolable products, while [{W(CO)4[C(OEt)]2}2-5,4,5′,4′-C8S3] (17, 13%) is the only identifiable product from the reaction with tungsten hexacarbonyl. We ascribe the low yield of 13% to steric congestion at the termini of the bent thiophene band as a result of the curvature of the linker DTT.
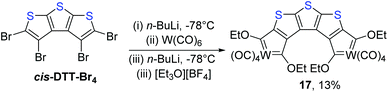 | ||
| Scheme 2 Preparation of a new tungsten(0) chelated FCC from a tetrabrominated dithienothiophene precursor with sulphur atoms in the up-up-up orientation. | ||
Spectroscopic characterization
| Complex | H4′ | H5′ | OEta | OEt | Bub | Et2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
|---|---|---|---|---|---|---|
| a Proton chemical shifts for the ethoxy fragments are reported with the first value being the chemical shift of the methylene group, and the second the chemical shift of the methyl group. The first set of methylene and methyl group resonances belongs to the ethoxy fragment coordinated through the C7 carbene, and the second set of values to the ethoxy fragment coordinated through the C6 carbene. b Proton chemical shifts for the CH2CH2CH2CH3 fragment are reported with the first value being the chemical shift of the first methylene group adjacent to the C5′ atom, followed by the second and third methylene group's values. The last chemical shift belongs to the methyl group. c Proton chemical shifts for the ethoxy fragment, coordinated through the C6′ carbene, are reported with the first value being the chemical shift of the methylene group, and the second the chemical shift of the methyl group. d Proton chemical shifts for the C5′-ethoxy fragment, reported with the first value being the chemical shift of the methylene group, and the second the chemical shift of the methyl group. e Proton chemical shifts for the (C4′CEt2)-ethyl fragments, reported with the first values being the chemical shifts of the methylene groups, and the second the chemical shift of the methyl groups. | ||||||
| 1 | 7.21 | 7.60 | 4.78, 1.70 and 4.72, 1.61 | |||
| 2 | 4.74, 1.68 and 4.69, 1.60 | 2.87, 1.71, 1.43, 0.97 | ||||
| 3 | 4.71, 1.67 and 4.70, 1.62 | |||||
| 4 | 8.91 | 4.75, 1.70 and 4.72, 1.62 | 5.34c, 1.86c | |||
| 5 | 7.20 | 7.71 | 4.54, 1.71 and 4.50, 1.61 | |||
| 6H | 7.56 | 4.50, 1.68 and 4.48, 1.62 | 2.82, 1.71, 1.44, 0.97 | |||
| 6Br | 4.51, 1.69 and 4.48, 1.61 | 2.82, 1.69, 1.44, 0.97 | ||||
| 7 | 4.54, 1.69 and 4.47, 1.59 | 5.06c, 1.69c, 4.12d, 1.44d | 2.39, 2.16, 0.71, 0.71 | |||
| 8 | 4.56, 1.71 and 4.48, 1.60 | |||||
| 9 | 7.31 | 7.55 | 4.78, 1.72 and 4.71, 1.61 | |||
| 11 | 4.77, 1.71 and 4.70, 1.61 | |||||
| 12 | 7.32 | 7.60 | 4.55, 1.73 and 4.50, 1.61 | |||
| 14Br | 4.55, 1.74 and 4.48, 1.60 | 2.88, 1.74, 1.44, 0.97 | ||||
| 14H | 7.48 | 4.55, 1.74 and 4.49, 1.61 | 2.88, 1.75, 1.44, 0.97 | |||
| 15 | 8.74 | 4.51, 1.74 and 4.48, 1.63 | 5.17c, 1.76c | |||
| 16 | 4.54, 1.72 and 4.47, 1.62 | |||||
| 17 | 4.62, 1.63 and 4.50, 1.53 | |||||
The metallacycle in each of the complexes 1–17 display two carbene ligands in different electronic environments due to their orientations with respect to the sulphur in the adjacent thiophene ring.
Comparing the monochelated biscarbene complexes of trans-TT (1 (Cr) and 5 (W)) to their analogous cis-TT carbene complexes, the H5′ resonances are ca. 0.15 (Cr) and 0.22 ppm (W) more downfield and the H4′ resonances ca. 0.20 (Cr) and 0.25 ppm (W) more upfield.32 This is a result of the larger extent of π-conjugation and more delocalised π-orbital electrons in trans-TT compared to cis-TT, allowing electron density to be withdrawn from the 5′-position throughout the trans-TT spacer in a carbene complex.36 The proton resonances are very similar when comparing the monochelated carbene complexes of trans-TT, (1 (Cr) and 5 (W)) to their analogous trans-DTT carbene complexes (9 (Cr) and 12 (W)). In the case of 1 and 5, the H5′ and H4′ resonances are slightly more downfield and upfield (ca. 0.1 ppm), respectively (Table 1).
The effect of the number of annulated rings can be estimated by comparing the H4 and H5 resonances for the thiophene (T) and up-up(-up) thienylene precursors, cis-TT and cis-DTT, with the monochelate biscarbene complex derivatives (Fig. 2). In the case of a single thienyl ring chelate carbene complex, T-[Cr],25 a significantly larger difference between H5 and H4 chemical shifts (ΔH5,4 in Fig. 2) in the complex compared to the precursor thiophene is indicative of polarisation in the thienyl ring. This we ascribe to the one thiophene double bond being involved in the delocalisation of electron density of the biscarbene chelate ring which has an adverse effect on electron delocalisation in the single thiophene ring. This is not the case for the furthest (uncoordinated) thiophene ring in trans-TT and trans-DTT biscarbene chelates. Hence, the polarisation in the thiophene ring decreases as the number of annulated thiophenes increase, so that ΔH5,4 increase in the order 9 < 1 < T-[Cr]. Moreover, the difference between the precursor absolute value for both H5 and H4, and the chelate complex derivative H5 and H4 values, also decreases as the number of annulated rings in the complex increases, so that ΔH4 and ΔH5 also increase in the order 9 < 1 < T-[Cr] (Fig. 2). Both trends support a lesser degree of electron delocalisation (and ring polarisation increase) in the order DTT > TT > T as the annulated ring number decreases.
 | ||
| Fig. 2 H4 and H5 chemical shift (ppm) differences observed for thiophene building blocks and their corresponding monochelate biscarbene complexes of chromium; T-[Cr],251 and 9. | ||
13C NMR data are summarized in Table 2. The carbene carbon chemical shifts of the metallacyclic biscarbene component, C7 and C6, are mostly unaffected by the type of annulated thienylene spacer present in the molecule, but affected by the position of attachment and the nature of the transition metal.
| Complex | C4 | C5 | C4′ | C5′ | Ccarb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Cco![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
OEtc | Bu/Et |
|---|---|---|---|---|---|---|---|---|
| a Carbene carbon chemical shift of C7 is reported first, followed by C6. b Carbon chemical shifts for the metal carbonyls are reported with the first two values being the chemical shifts of the carbonyls trans to the carbene carbons, and the third the chemical shift of the carbonyls cis to the carbene carbons. c Carbon chemical shifts for the ethoxy fragment are reported with the first value being the chemical shift of the methylene group, and the second the chemical shift of the methyl group, respective for the C7 (first set of values) and C6 (second set of values) carbene fragments. d Carbon chemical shifts for the CH2CH2CH2CH3 fragment are reported with the first value being the chemical shift of the first methylene group after the C5′ atom, followed by the second and third methylene group's values. The last chemical shift belongs to the methyl group. e Assignments could not be made unambiguously. f Carbon chemical shifts for the C6′-carbene fragment. The carbon chemical shifts for the pentacarbonyls are reported with the first value being the chemical shift of the carbonyl trans to the carbene carbon, and the second the chemical shift of the carbonyls cis to the carbene carbon. Ethoxy fragment resonances are reported with the first value being the chemical shift of the methylene group, and the second the chemical shift of the methyl group. g Carbon chemical shifts for the C5′-ethoxy fragment, reported with the first value being the chemical shift of the methylene group, and the second the chemical shift of the methyl group. h Carbon chemical shifts for the (C4′CEt2)-fragment. The first value is the chemical shift of the C4′CEt2 carbon, the second value the methylene and the third the methyl chemical shift. i Two carbon chemical shifts are observed for the cis metal carbonyl ligands. | ||||||||
| 1 | 157.0 | 163.4 | 120.0 | 132.8 | 314.4, 309.2 | 243.0 and 242.9, 227.8 | n.o., 15.1; 76.4, 15.1 | |
| 2 | 157.2 | 160.8 | 101.9 | 148.3 | 315.0, 308.2 | 243.2 and 242.8, 227.8 | n.o., 15.0; 76.4, 15.0 | 30.3d, 32.5d, 22.1d, 13.7d |
| 3 | 157.8 | 165.0 | 314.6, 310.0 | 244.8 and 242.4, 227.5 | n.o., 15.1; n.o., 15.1 | |||
| 4 | 314.6, 310.9, 321.7f | 243.3 and 243.0, 227.8, 222.9f, 216.8f | n.o., 15.1; n.o., 15.0; n.o.f, 15.8f | |||||
| 5 | 161.3 | 169.6 | 120.4 | 133.0 | 286.8, 282.7 | 220.5 and 220.2, 212.5 | 79.7, 14.8; 79.5, 14.7 | |
| 7 | 161.9 | 163.6 | 147.1 | 165.4 | 287.5, 278.8, 341.9f | 221.7 and 219.2, 212.4, 200.9f, 197.2f | 79.7, 14.8; 79.1, 14.8; 81.5f, 14.7f; 70.1g, 14.7g | 70.7h, 26.8h, 9.1h |
| 11 | 157.6 | 162.4 | 314.4, 308.0 | 243.9 and 242.7, 227.6 | 76.7, 15.0; n.o., 15.0 | |||
| 16 | 162.1 | 169.0 | 286.0, 280.7 | 220.4 and 219.7, 212.0 | 79.7, 14.7; 79.6, 14.7 | |||
| 17 | 162.1 | 169.0 | 296.5, 282.9 | 219.4 and 216.8, 212.4 and 210.7i | 80.3, 14.8 and 14.7; 79.5, 14.9 and 14.9 | |||
Considering the triscarbene complex of trans-TT (4), the C6 and C6′ carbene carbon chemical shifts are more downfield compared to the analogous cis-TT carbene complex.32 Comparing the metallacyclic tetracarbonyl biscarbene fragment of 4 to its intramolecular C5′ pentacarbonyl carbene fragment, the carbene signals are upfield and the carbonyl carbon signals downfield.
The C7 carbene carbon resonance is more upfield in the chromium bischelated carbene complex of trans-TT (3), compared to the analogous cis-TT carbene complex.32 The same effect is seen in comparing 16 (trans-DTT) with 17 (cis-DTT). The larger extent of π-conjugation in trans-TT and trans-DTT, compared to their constitutional isomers, permits more electron density to be delocalised to the C7 carbene carbons of 3 and 16. In addition, the linear structure of 16 compared to the bent molecular structure of 17 results in better pπ-orbital overlap in the conjugated pathway of 16 (Fig. 3). As a result, greater shielding of the carbene carbon atoms in 16 is found, compared to those in 17. Steric congestion in 17 results in the chemical non-equivalence of the two cis carbonyl ligands (disruption of the symmetry) and two separate resonances, at 212.4 and 210.7 ppm, are observed instead of the expected one. The same duplication is observed in the methyl resonances of C7OEt and C6OEt. Chemical shift assignments of C2 and C3 could not be made unambiguously for the compounds.
Single crystal X-ray molecular structures
Single crystals suitable for X-ray diffraction were obtained from saturated solutions of either DCM (1–3, 5) or benzene (11, 16), each layered with hexane. X-ray diffraction studies confirmed their molecular structures (Fig. 4) and selected bond lengths, angles and torsion angles are given in Table 3. The atom numbers employed are similar to the NMR assignments. A single crystal of 7 could be isolated, but the diffraction data set is of low resolution and therefore not included. However, the atom connectivity in the molecule can be deduced from the structure (see section S5, ESI†). | ||
| Fig. 4 The molecular structures of 1–3, 5, 11 and 16 with the atomic displacement ellipsoids shown at the 50% probability level. | ||
| Complex | 1 | 2 | 3 | 5 | 11 | 16 |
|---|---|---|---|---|---|---|
| a Average bond length. b First mean plane drawn through C2, C3, C4 and C5, and the second through M, C7carb, O7, C6carb and O6. c First set reported for M1 and the second for M2. In the case of 3 only one set of values is reported, as the values are identical due to the symmetry of the molecule. | ||||||
| Bond lengths | ||||||
| M–C7carb | 2.029(1) | 2.029(5) | 2.030(2) | 2.155(3) | 2.020(5), 2.025(5) | 2.161(6), 2.166(5) |
| M–C6carb | 2.040(2) | 2.053(5) | 2.037(2) | 2.169(4) | 2.041(6), 2.029(4) | 2.175(4), 2.161(6) |
| C7carb–OEt | 1.322(2) | 1.318(6) | 1.325(2) | 1.340(4) | 1.308(7), 1.325(7) | 1.326(7), 1.323(5) |
| C6carb–OEt | 1.324(2) | 1.326(6) | 1.324(3) | 1.321(4) | 1.311(6), 1.323(6) | 1.316(6), 1.316(5) |
M–COtrans to CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
1.918(2) | 1.918(5) | 1.922(2) | 2.061(4) | 1.918(6), 1.913(6) | 2.053(5), 2.060(5) |
M–COtrans to carb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
1.878(2) | 1.884(6) | 1.890(2) | 2.021(4) | 1.897(6), 1.887(6) | 2.021(5), 2.021(6) |
| C7carb–C4 | 1.443(3) | 1.451(7) | 1.450(3) | 1.438(6) | 1.454(8), 1.456(7) | 1.461(6), 1.463(8) |
| C6carb–C5 | 1.438(2) | 1.444(7) | 1.441(3) | 1.447(5) | 1.449(7), 1.443(7) | 1.449(8), 1.449(7) |
| C2–C3 | 1.383(3) | 1.379(7) | 1.386(3) | 1.378(6) | 1.390(8), 1.394(8) | 1.401(6), 1.390(8) |
| C3–C4 | 1.420(2) | 1.418(7) | 1.418(3) | 1.425(5) | 1.418(7), 1.416(7) | 1.411(7), 1.420(7) |
| C4–C5 | 1.384(2) | 1.377(6) | 1.382(3) | 1.388(5) | 1.385(8), 1.396(8) | 1.391(8), 1.392(6) |
| S–C2 | 1.736(2) | 1.732(5) | 1.732(2) | 1.739(3) | 1.722(6), 1.738(6) | 1.728(6), 1.732(4) |
| S–C5 | 1.732(2) | 1.742(5) | 1.739(2) | 1.730(4) | 1.737(6), 1.724(5) | 1.732(4), 1.730(6) |
| Bond angles | ||||||
| (O)Ccis,axial–M–Ccis,axial (O) | 10.48(8) | 10.0(2) | 8.18(9) | 3.8(2) | 13.2(2), 13.3(2) | 3.1(2), 5.1(2) |
| C6carb–M–C7carb | 81.67(6) | 81.7(2) | 81.80(8) | 77.3(1) | 82.5(2), 82.3(2) | 77.7(2), 77.7(2) |
| M–C7carb–O7 | 137.7(1) | 137.6(3) | 138.8(1) | 137.2(2) | 137.8(4), 138.4(4) | 137.1(3), 136.5(3) |
| M–C6carb–O6 | 137.6(1) | 137.3(3) | 137.8(1) | 136.8(3) | 139.6(4), 137.7(3) | 137.6(3), 137.6(3) |
| M–C7carb–C4 | 113.4(1) | 113.4(3) | 113.1(1) | 114.9(2) | 113.7(3), 113.4(3) | 114.9(3), 114.5(3) |
| M–C6carb–C5 | 112.3(1) | 111.8(3) | 112.3(1) | 113.3(2) | 110.8(4), 112.2(3) | 113.1(3), 112.9(3) |
| O7–C7carb–C4 | 108.8(1) | 109.0(4) | 108.1(2) | 107.9(3) | 108.3(4), 108.1(4) | 107.9(4), 109.0(4) |
| O6–C6carb–C5 | 110.1(1) | 110.9(4) | 109.7(2) | 109.9(3) | 109.5(4), 109.9(4) | 109.3(4), 109.4(4) |
| Torsion angles | ||||||
| M–C7carb–C4–C5 | 2.2(2) | −5.4(5) | 1.6(2) | −4.6(4) | −3.2(6), 2.4(6) | 0.9(5), 8.8(5) |
| M–C6carb–C5–C4 | −0.6(2) | −1.3(5) | −3.9(2) | 2.5(4) | 2.0(6), 0.2(6) | −0.9(6), −3.6(6) |
| O7–C7carb–C4–C5 | −180.0(1) | 175.1(4) | −179.3(2) | 178.0(3) | −179.0(5), −173.8(4) | −179.9(4), −169.8(4) |
| O6–C6carb–C5–C4 | 179.3(1) | 178.5(4) | 179.4(2) | −178.1(3) | −179.7(5), −176.0(4) | −178.9(4), 179.2(4) |
| Angle between two mean planesb | 3.28 | 4.66 | 3.71 | 6.11 | 3.83, 4.42 | 0.61, 9.65 |
Crystal structures of the octahedral chelated ethoxycarbene complexes have similar structural features. The octahedral metal tetracarbonyl fragment is attached to the spacer through two ethoxycarbene carbons (C7 and C6). The ethyl groups (C7 and C6) and metal moiety are on the same side of the ((CO)4M)Ccarb–O(Et) bond (C7carb–O7(Et) and C6carb–O6(Et) respectively), ensuing the favoured anti-isomer orientation.38–41
To determine if the thienylene spacer and metallacyclic biscarbene component (carbene carbon atoms, metal and ethoxy fragments) are in the same plane, a mean plane is drawn through C2, C3, C4 and C5, and a second through M, C7carb, O7, C6carb and O6. All of the crystal structures exhibited a near planar arrangement, as indicated by the angle measured between the two planes (Table 3). The larger tungsten metal in 5 and 16 accounts for the largest deviation from planarity of the molecules and the angle between the two mean planes is determined as 6.11 and 9.65° (on one side of the molecule), respectively. All the structures in Fig. 4, except for the slightly curved monochelated complexes 1, 2 and 5, adopt a linear arrangement, as a result of their annulated thiophene rings with sulphur atoms arranged in the up-down(-up) positions along with the presence of condensed chelates biscarbene fragments. This is not the case in the bent structures of annulated thiophenes with the sulphur atoms on the same side (compare also Fig. 3).32 The metal centers have an octahedral arrangement of ligands with small deviations in the bond angles caused by the chelate ring/s. The two cis carbonyl ligands axially arranged (and trans to each other) are slightly bent towards the inside of the chelated rings. The (O)Ccis,axial–M–Ccis,axial(O) bond angles are measured to indicate the degree of deviation from linearity (Table 3). Another bond angle that is affected is the bite angle (C6carb–M–C7carb) of coordination for the chelate ring, and they are smaller than 90°. The M–Ccarb bond lengths (with Ccarb as C7carb or C6carb) are all comparable within experimental error for the chromium and tungsten carbene complexes, respectively, with the M–C6carb bond lengths mostly significantly longer compared to M–C7carb. The Ccarb–OEt averaged bond lengths (C7carb–OEt (Cr: 1.320(7); W: 1.330(6)) and C6carb–OEt (Cr: 1.320(5); W: 1.318(6)) are independent of the type of metal or annulated thienylene present in the molecule (Table 3). The same principle accounts for the bond lengths of the bonds connecting the carbene carbons to the annulated thienylene (C7carb–C4 and C6carb–C5).
Ethoxycarbene complexes have characteristic carbene carbon angles of 130° (M–Ccarb–O), 125 (M–Ccarb–C5) and 105° (O–Ccarb–C5).38–41 In the case of chelated ethoxycarbene complexes the M–Ccarb–O bond angle is increased to an average magnitude of 137.8(3)°, the M–C7carb/C6carb–C5/C4 bond angle significantly decreased to 113.2°(3) and the O–Ccarb–C5/C4 bond angle increased to 109.1(4)° because of the metallacycle ring strain effect on coordination (Table 3).
Cyclic voltammetry and molecular orbital calculations
Cyclic voltammetry (CV) and linear sweep (LSV) experiments were performed for 1, 3, 11, 16, 18–21 (Fig. 5). The choice of compounds for electrochemical studies was justified by the aim to investigate the effect of the following on the redox processes; monocarbene vs. mononuclear chelated biscarbene (e.g.18vs. 1), mononuclear chelated carbene vs. dinuclear chelated carbene complexes (e.g.1vs. 3), Cr vs. W (11vs. 16) and different thienylene spacers (condensed thiophenes with the sulphur atoms in the up-up (19) vs. up-down (3) vs. up-up-up (20) vs. up-down-up (e.g.21) positions). The electrochemical data are summarized in Table 4 and Table S7, ESI† and the CVs for the reduction processes are displayed in Fig. 6. Ferrocene (FcH) was used as internal standard for 16, 18, 20 and 21, and decamethylferrocene ([Fe(η5-C5Me5)2]) was used for the remainder of the compounds (to avoid overlap observed with FcH). The [Fe(η5-C5Me5)2]0/+1 couple was referenced to the FcH0/+1 couple (E°′ = 0.00 V), to enable comparison with the results of the compounds measured with FcH as internal standard. | ||
| Fig. 6 CVs of the overlapping reduction events of (a) 18 (blue) and 1 (red); (b) 19 (blue) and 3 (red), including LSVs; (c) 11 (blue) and 16 (red), and (d) 21 (blue) and 20 (red). | ||
| Compound | Reduction (R) | Compound | Reduction (R) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R1 | R2 | R3 | R4 | ||
| Only unambiguously identifiable waves are reported.a No Epa detected.b Fifth reduction observed with Epc = −2.19 V. | |||||||||
| 1 | E pa = −1.41, Epc = −1.50, E°′ = −1.45, ΔEp = 0.09 | E pa = −1.85, Epc = −1.92, E°′ = −1.89, ΔEp = 0.07 | 18 | E pa = −1.60, Epc = −1.70, E°′ = −1.65, ΔEp = 0.10 | |||||
| 3 | E pa = −1.17, Epc = −1.24, E°′ = −1.20, ΔEp = 0.07 | E pa = −1.36, Epc = −1.41, E°′ = −1.38, ΔEp = 0.05 | E pa = −2.06, Epc = −2.13, E°′ = −2.10, ΔEp = 0.07 | E pc = −2.40a | 19 | E pa = −1.32, Epc = −1.39, E°′ = −1.35, ΔEp = 0.06 | E pa = −1.57, Epc = −1.64, E°′ = −1.60, ΔEp = 0.07 | E pc = −2.20a | |
| 11 | E pa = −1.23, Epc = −1.30, E°′ = −1.27, ΔEp = 0.07 | E pa = −1.35, Epc = −1.44, E°′ = −1.40, ΔEp = 0.09 | E pc = −1.99a | E pc = −2.17a | 20 | E pa = −1.64, Epc = −1.71, E°′ = −1.68, ΔEp = 0.07 | |||
| 16 | E pa = −1.07, Epc = −1.12, E°′ = −1.10, ΔEp = 0.05 | E pa = −1.19, Epc = −1.24, E°′ = −1.22, ΔEp = 0.05 | E pa = −1.74, Epc = −1.79, E°′ = −1.76, ΔEp = 0.05 | E pc = −2.07a | 21 | E pa = −1.54, Epc = −1.75, E°′ = −1.64, ΔEp = 0.21 | E pc = −2.30a | ||
The CV data for the oxidation processes (O) are summarized in Table S7 (ESI†). The observed oxidation processes are metal-based21,36 and predominantly chemically and electrochemically irreversible, hence limited information could be extracted from the results. The resulting oxidations processes cannot be assigned unambiguously as Cr0/I up to CrV/VI couples are possible, as well as the oxidation of electrochemically produced reactive Cr0/I and CrI/II intermediates. The large irreversible oxidation processes observed, range from two to six electron processes and are discussed in the ESI section S7.†
The reduction processes (R) are centred on the carbene double bond (M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), to yield the radical anion; M–C˙−.21,36 The carbene double bond reduction is reversible for tungsten carbene complexes on CV time scale. For chromium carbene complexes, this reduction varies between reversible/quasi-/irreversible reductions42 due to the fast decomposition rate of the radical anion product.43 In the case of 18 the reduction is irreversible as ΔEp is 100 mV (Table 4) and the ipc/ipa current ratio is far from unity. The high reactivity/subsequent decomposition that is associated with the Cr–C˙− species, results in the low current ratio observed.42 In aprotic solvents, the radical anion that is generated is extensively unstable and subsequent reactions destroy this electrochemically generated species quickly. The Cr
C), to yield the radical anion; M–C˙−.21,36 The carbene double bond reduction is reversible for tungsten carbene complexes on CV time scale. For chromium carbene complexes, this reduction varies between reversible/quasi-/irreversible reductions42 due to the fast decomposition rate of the radical anion product.43 In the case of 18 the reduction is irreversible as ΔEp is 100 mV (Table 4) and the ipc/ipa current ratio is far from unity. The high reactivity/subsequent decomposition that is associated with the Cr–C˙− species, results in the low current ratio observed.42 In aprotic solvents, the radical anion that is generated is extensively unstable and subsequent reactions destroy this electrochemically generated species quickly. The Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond reduction of 18 represents a one-electron reduction as the ipc value of R1 match the ipa value of FcH (known to signify a one-electron redox process, Fig. 6(a)).
C double bond reduction of 18 represents a one-electron reduction as the ipc value of R1 match the ipa value of FcH (known to signify a one-electron redox process, Fig. 6(a)).
During a one-electron transfer process, alkene reduction occurs at far negative potentials and conjugated alkenes at slightly larger potentials.42 Redox potentials are considered a good measure of the tendency of a system to undergo reduction (acquire electrons).21 The first reduction (R1) potentials of the monocarbene complexes 18, 20 and 21 are around −1.66 V vs. FcH0/+1, which is far more negative compared to the remaining chelated carbene complexes (Table 4, R1). Comparing R1 of 18 to R1 of the chromium thiophene monocarbene complex (−1.76 V), the latter is found at an even greater negative potential.42
Two separate reductions are observed in the CV of 1 (Fig. 6(a)). R1 can be ascribed as a quasi-reversible reduction of Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C as ΔEp = 90 mV and ipc/ipa = 2. R1 is at −1.45 V, which is less negative compared to the R1 value of 18. R2 represents an irreversible reduction of Cr
C as ΔEp = 90 mV and ipc/ipa = 2. R1 is at −1.45 V, which is less negative compared to the R1 value of 18. R2 represents an irreversible reduction of Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C as the current ratio ipc/ipa is ca. 4. R1 and R2 represent a one-electron reduction as their ipc values match the ipa value of the [Fe(η5-C5Me5)2]0/+1 couple (one-electron redox process, Fig. 6(a)). Comparing the chelated biscarbene complex to a standard thiophene biscarbene complex, R1 of the latter is at −1.85 V (0.4 V more negative).42 This is in sharp contrast to the results found for chelated monocarbene complexes, where they are reduced at more negative potentials compared to non-chelated carbene complexes in the same study.21 Four one-electron reductions are observed in the CV of 3 (Fig. 6(b)). R1 and R2 are overlapping, but still distinguishable and are considered quasi-reversible reductions as their ipc/ipa current ratios are around 2. R3 and R4 are irreversible reductions with the current ratio of R3 ca. 4 and that of R4 undetermined, as the reduction occurs at the edge of the solvent potential window.
C as the current ratio ipc/ipa is ca. 4. R1 and R2 represent a one-electron reduction as their ipc values match the ipa value of the [Fe(η5-C5Me5)2]0/+1 couple (one-electron redox process, Fig. 6(a)). Comparing the chelated biscarbene complex to a standard thiophene biscarbene complex, R1 of the latter is at −1.85 V (0.4 V more negative).42 This is in sharp contrast to the results found for chelated monocarbene complexes, where they are reduced at more negative potentials compared to non-chelated carbene complexes in the same study.21 Four one-electron reductions are observed in the CV of 3 (Fig. 6(b)). R1 and R2 are overlapping, but still distinguishable and are considered quasi-reversible reductions as their ipc/ipa current ratios are around 2. R3 and R4 are irreversible reductions with the current ratio of R3 ca. 4 and that of R4 undetermined, as the reduction occurs at the edge of the solvent potential window.
Compared to 3, 19 has two less-overlapping, one-electron, quasi-reversible peaks (R1 and R2) at more negative potentials. R3 is an irreversible reduction consisting of at least two devoted one-electron transfer processes (see linear sweep voltammetry (LSV), Fig. 6(b)). Compounds 11 and 16 (chromium and tungsten analogues, respectively) have very similar CVs (Fig. 6(c)), with the reductions of 11 at more negative potentials. The CVs show two overlapping one-electron reductions (R1 and R2, quasi-reversible) and two or three one-electron irreversible reductions.
Comparing 20 and 21 (cis-DTT vs. trans-DTT spacer), the CVs are analogous as both compounds show an one-electron quasi-reversible reduction at more or less the same reduction potential, Fig. 6(d). In the case of 21, a second reduction is observed with Epc = −2.30 V, indicating the formation of 21−2.
Considering the chromium bis-chelated tetracarbene complexes, 3, 11 and 19; 19 with a cis-TT spacer stabilized the Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond more towards reduction (more negative reduction potential). The order from most stabilized is, 19 > 11 (trans-DTT) > 3 (trans-TT), with ca. 80 mV difference between the compounds. When comparing the chromium and tungsten analogous complexes, 11 and 16 respectively, 11 is reduced at a potential 170 mV more negative. When comparing the cis-DTT and trans-DTT analogous complexes, 20 and 21 respectively, 20 is reduced at a potential 40 mV more negative.
C bond more towards reduction (more negative reduction potential). The order from most stabilized is, 19 > 11 (trans-DTT) > 3 (trans-TT), with ca. 80 mV difference between the compounds. When comparing the chromium and tungsten analogous complexes, 11 and 16 respectively, 11 is reduced at a potential 170 mV more negative. When comparing the cis-DTT and trans-DTT analogous complexes, 20 and 21 respectively, 20 is reduced at a potential 40 mV more negative.
Molecular orbital and spin density calculations are performed at the dispersion corrected B3LYP-D3/def2-SVP level for 1, 3, 11, 18 and 19 to assist with assigning the CV reduction processes and their relative orders (Fig. 7). For the monocarbene complex 18, the LUMO is mainly centered on the vacant pz-atomic orbital of the carbene carbon atom and the additional electron gained upon reduction (R1) occupies this orbital (computed spin density of 0.48e).
 | ||
| Fig. 7 Summary of spin-density and molecular orbital calculations performed for 18, 1, 3, 19 and 11. All data have been computed at the B3LYP-D3/def2-SVP level. | ||
The orbitals associated with carbene complexes are delocalised over the metal, carbene carbon, and oxygen heteroatom of the ethoxy fragment.42 The Cr–C˙− species are envisioned as being stabilized by distributing charge and the radical over the conjugated ligand system. From the LUMO of 18, it is clear that the carbene carbon is stabilized with significant contributions of the conjugated carbon atoms and less so by the lone pair on the adjacent sulphur atom of the trans-TT spacer (Fig. 7). The greater negative reduction potential of 18 is reflected in the energy of its LUMO, which is the highest of the entire series. In the case of the mono-chelated biscarbene complex 1, the LUMO is almost equally distributed over both carbene carbon atoms. Subsequent reductions confirm that the two electrons are placed on both carbene carbon atoms (see spin density of 1•− and HOMO of the reduced 1−2 system, Fig. 7). Reductions therefore occur on both carbene carbon centers simultaneously, during the first and second reduction (R1 and R2) and are delocalised over the five-membered metallacyclic ring. According to the more stabilized LUMO of 1, this complex is reduced at lower negative potentials than 18. The LUMO of bis-chelated tetracarbene complex 3 is localized on all carbene carbon atoms, with a larger contribution on the carbene carbon atoms adjacent to the sulphur atoms (C6), as well as on the sulphur atoms in the cis-TT spacer. As a result, the first electron is delocalised over the carbene carbon atoms adjacent to the sulphur atoms. These carbene carbons lie in the conjugated pathway, where they are linked through the thienothienyl with the metal fragments. The second reduction electron leads to the open-shell singlet specie 3−2 where the unpaired electrons are delocalised across the remote carbene carbon atoms. The third and fourth electrons will presumably complete both singly occupied orbitals to produce a species similar to the reduced closed-shell 1−2 system. The reductions are therefore stepwise and site specific. R1 of 3 is assigned to the initial reduction that takes place simultaneously at the two carbene double bonds that neighbour the sulphur atoms in trans-TT. R2 is then assigned as the second reduction that takes place at the carbene double bonds remote to the sulphur atoms in trans-TT. R3 and R4 complete the occupation of open-shell specie 3−2. Compound 3 exhibits the most stabilized LUMO energy and the second least negative R1 potential (Fig. 7 and Table 4).
Compound 19 and 11 do not show significant differences in their LUMOs and their behaviors are almost identical to that of 3. From the LUMO visualisation of 19, it is clear that the sulphur atoms in the cis-TT spacer do not contribute to the stabilization of the carbene carbon atoms (Fig. 7). Compared to 3 and 11, 19 has a higher LUMO energy accompanying the more negative reduction potential observed.
From the LUMO visualisation of 11, the longer conjugated spacer (trans-DTT) shows contribution of the sulphur atoms adjacent to the carbene carbon atoms, but not the central sulphur atom. A second aspect to consider for the ease of the first reduction in the tetracarbene complexes, is the number of thiophene rings in the linear, conjugated spacer. While ELUMO: 3 (−3.50 eV) < 11 (−3.45 eV), the first reductions (R1) are: 3 (E°′ = −1.20 eV) > 11 (−1.27 eV) while the second reductions are more comparable (Table 4).
In this study, no evidence for the formation of mixed-valence electrochemical intermediates showing metal–metal communication is seen upon oxidation or reduction, as observed for ferrocenyl FCCs of chromium.44 Rather the complexes behave as simple carbene complexes that accept electrons in their LUMOs, which are more or less conjugated with the thiophene moiety. The LUMO level correlates perfectly with the reduction potential and are indicative of the extent of this conjugation involving the lone pair/s into the empty pz atomic orbital of the carbene carbon atom. A similar result was found for related extended π-conjugated Fischer biscarbene complexes.45
Conclusions
Chelated carbene complexes were synthesized from using tetrabromothienylene starting materials. Selective stepwise lithiation with the addition of two equivalents of metal hexacarbonyl, in-between the two dilithiations, followed by alkylation yielded new examples of the rare class of chelated multicarbene complexes of the Fischer-type. The reaction conditions chosen for the preparative route allowed for the targeted bis-chelated dinuclear tetracarbene complexes as major products. These complexes display either a linear overall molecular geometry when employing the up-down(-up) trans-TT or trans-DTT spacers (e.g.3, 8, 11 and 16), or the bent/semi-circular molecular geometry of the complexes featuring the up-up-up cis-DTT spacer between the terminal metallacyclic bicarbene moieties in 17, similar to the up-up analogue 19.32 The two carbene ligands in the chelate ring are clearly electronically different as was confirmed by the DFT calculations and experimentally by different chemical shifts in the corresponding 1H and 13C NMR spectra and the bond distances around the carbene carbons in the solid state structures.Cyclic voltammetry experiments indicated that the reduction of the chelated carbene complexes result in the negative charge being delocalised over the entire metallacyclic ring (all carbene carbon atoms). The chelated carbene complexes reductions are more facile compared to monodentate carbene complexes, and their LUMO energies lower. In the case of chelated carbene complexes with linear conjugated annulated thienylene spacers (trans-TT and trans-DTT), the electron delocalisation of the negative charge is also over the thienylene carbons and adjacent sulphur atom/s (increasing electron density on the heterocyclic part). The sulphur atom involvement in electron delocalisation contributes to the LUMO of the complexes, causing reduction at less negative potentials, compared to complexes with bent non-conjugated cis-TT and cis-DTT spacers where the contribution of the sulphur atoms is absent and the formed radical-anions do not adopt a planar arrangement with a co-planar carbene carbon.42,43 In summary, chelation of the carbene ligands allows for the conjugation of the empty carbene carbon pz orbitals with the aromatic thiophene rings in the formed metallacycles. This effect is enhanced when the ring systems are linear as opposed to bent when the sulphur atoms are oriented cis to each other.
No mixed valence species formed during the stepwise reductions of chelated carbene complexes and no evidence of through-bond electronic communication is observed between the metal–carbene fragments. The individual oxidation and reduction waves are split due only to electrostatic effects.36 This is in contrast to the mixed valence species observed for ferrocenyl carbene complexes.44
Experimental
General
All operations were carried out using standard Schlenk techniques or vacuum line techniques under an inert atmosphere of nitrogen or argon, using oven-dried glassware. Silica gel 60 (particle size 0.063–0.20 mm) was used as resin (stationary phase) for all column chromatography separations.Chemical reagents and solvents
Triethyloxonium tetrafluoroborate was prepared according to literature procedure and stored in diethyl ether under Ar (g).46 Boron trifluoride etherate was distilled before use. Anhydrous solvents, which were degassed through bubbling N2, were used for experimental procedures. THF and diethyl ether were distilled over sodium wire and benzophenone under N2 (g) atmosphere, hexane and benzene over sodium wire and DCM over CaH2. Other chemicals were used as they were commercially supplied by Sigma Aldrich and Strem Chemicals. The n-BuLi used in syntheses, was from a stock 1.6 M solution in hexane. Compounds 4,4′,5,5′-tetrabromo-thieno[3,2-b]thiophene (trans-TT-Br4), 4,4′,5,5′-tetrabromo-dithieno[2,3-b;3′,2′-d]thiophene (cis-DTT-Br4) and 4,4′,5,5′-tetrabromodithieno[3,2-b;2′,3′-d]thiophene (trans-DTT-Br4) were prepared according to literature procedures.47–49 Compounds 18,5019,3220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 and 21
51 and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 were prepared according to literature procedures, for comparative cyclic voltammetry studies.
35 were prepared according to literature procedures, for comparative cyclic voltammetry studies.
Synthesis and characterization of Fischer carbene complexes
1: UV-Visλmax (CH2Cl2)/nm 618 and 373, from a sample with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 of 1
0.6 of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (not quantitative). FT-IRνCO (hexane)/cm−1 2017m (A1(1)), 1962s (B1), 1947m (A1(2)), 1895m (B2). 1H NMRδ1H (300.13 MHz; CDCl3; Me4Si) 7.60 (1 H, d, 3J5′,4′ 5.2, H5′), 7.21 (1 H, d, 3J4′,5′ 5.2, H4′), 4.78 (2 H, q, 3J 7.1, C7CH2), 4.72 (2 H, q, 3J 7.1, C6CH2), 1.70 (3 H, t, 3J 7.1, C7CH3), 1.61 (3 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 314.4 (C7carb), 309.2 (C6carb), 243.0 and 242.9 (COtrans), 227.8 (COcis), 163.4 (C5), 157.0 (C4), 145.7 and 127.7 (C3 and C2), 132.8 (C5′), 120.0 (C4′), n.o. (C7CH2), 76.4 (C6CH2), 15.1 (C7CH3 and C6CH3).
2 (not quantitative). FT-IRνCO (hexane)/cm−1 2017m (A1(1)), 1962s (B1), 1947m (A1(2)), 1895m (B2). 1H NMRδ1H (300.13 MHz; CDCl3; Me4Si) 7.60 (1 H, d, 3J5′,4′ 5.2, H5′), 7.21 (1 H, d, 3J4′,5′ 5.2, H4′), 4.78 (2 H, q, 3J 7.1, C7CH2), 4.72 (2 H, q, 3J 7.1, C6CH2), 1.70 (3 H, t, 3J 7.1, C7CH3), 1.61 (3 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 314.4 (C7carb), 309.2 (C6carb), 243.0 and 242.9 (COtrans), 227.8 (COcis), 163.4 (C5), 157.0 (C4), 145.7 and 127.7 (C3 and C2), 132.8 (C5′), 120.0 (C4′), n.o. (C7CH2), 76.4 (C6CH2), 15.1 (C7CH3 and C6CH3).
2: UV-Visλmax (CH2Cl2)/nm 614 and 366, compound decomposed in solution (not quantitative). FT-IRνCO (hexane)/cm−1 2018m (A1(1)), 1962s (B1), 1947m (A1(2)), 1896m (B2). 1H NMRδ1H (300.13 MHz; CDCl3; Me4Si) 4.74 (2 H, q, 3J 6.8, C7CH2), 4.69 (2 H, q, 3J 7.0, C6CH2), 1.68 (3 H, t, 3J 6.8, C7CH3), 1.60 (3 H, t, 3J 7.0, C6CH3), 2.87 (2 H, t, 3J 7.5, CH2CH2CH2CH3), 1.76–1.67 (2 H, m, CH2CH2CH2CH3), 1.50–1.37 (2 H, m, CH2CH2CH2CH3), 0.97 (3 H, t, 3J 7.3, CH2CH2CH2CH3). 13C NMRδ13C (75.468 MHz; CDCl3; Me4Si) 315.0 (C7carb), 308.2 (C6carb), 243.2 and 242.8 (COtrans), 227.8 (COcis), 160.8 (C5), 157.2 (C4), 147.4 and 124.0 (C3 and C2), 148.3 (C5′), 101.9 (C4′), n.o. (C7CH2), 76.4 (C6CH2), 15.0 (C7CH3 and C6CH3), 30.3 (CH2CH2CH2CH3), 32.5 (CH2CH2CH2CH3), 22.1 (CH2CH2CH2CH3), 13.7 (CH2CH2CH2CH3).
3: UV-Visλmax (CH2Cl2)/nm 683 (ε/dm3 mol−1 cm−1 9770), 453 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170), 393 (28
170), 393 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240). FT-IRνCO (hexane)/cm−1 2018m (A1(1)), 1962s (B1), 1947m (A1(2)), 1895m (B2). νCO (DCM)/cm−1 2010m (A1(1)), 1959s (B1), 1938m (A1(2)), 1884m (B2). δ1H (400.13 MHz; CDCl3; Me4Si) 4.71 (4 H, q, 3J 7.1, C7CH2), 4.70 (4 H, q, 3J 7.1, C6CH2), 1.67 (6 H, t, 3J 7.1, C7CH3), 1.62 (6 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 314.6 (C7carb), 310.0 (C6carb), 244.8 and 242.4 (COtrans), 227.5 (COcis), 165.0 (C5), 157.8 (C4), 131.6 and 129.2 (C3 and C2), n.o. (C7CH2 and C6CH2), 15.1 (C7CH3 and C6CH3). HR-MSm/z (C26H20O12S2Cr2, 692.55 g mol−1) calculated: 662.9179, found: 662.9341 (5%, [M − H − CO]−), calculated: 634.9230, found: 634.9350 (5%, [M − H − 2CO]−).
240). FT-IRνCO (hexane)/cm−1 2018m (A1(1)), 1962s (B1), 1947m (A1(2)), 1895m (B2). νCO (DCM)/cm−1 2010m (A1(1)), 1959s (B1), 1938m (A1(2)), 1884m (B2). δ1H (400.13 MHz; CDCl3; Me4Si) 4.71 (4 H, q, 3J 7.1, C7CH2), 4.70 (4 H, q, 3J 7.1, C6CH2), 1.67 (6 H, t, 3J 7.1, C7CH3), 1.62 (6 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 314.6 (C7carb), 310.0 (C6carb), 244.8 and 242.4 (COtrans), 227.5 (COcis), 165.0 (C5), 157.8 (C4), 131.6 and 129.2 (C3 and C2), n.o. (C7CH2 and C6CH2), 15.1 (C7CH3 and C6CH3). HR-MSm/z (C26H20O12S2Cr2, 692.55 g mol−1) calculated: 662.9179, found: 662.9341 (5%, [M − H − CO]−), calculated: 634.9230, found: 634.9350 (5%, [M − H − 2CO]−).
4: 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 8.91 (1 H, s, H4′), 4.75 (2 H, q, 3J 7.0, C7CH2), 4.72 (2 H, q, 3J 7.1, C6CH2), 5.34 (2 H, q, 3J 7.0, C6′CH2), 1.70 (3 H, t, 3J 7.0, C7CH3), 1.62 (3 H, t, 3J 7.0, C6CH3), 1.86 (3 H, t, 3J 7.0, C6′CH3). 13C NMRδ13C (75.468 MHz; CDCl3; Me4Si) 314.6 (C7carb), 310.9 (C6carb), 321.7 (C6′carb), 243.3 and 243.0 ((CO)4 trans), 227.8 ((CO)4 cis), 222.9 ((CO)5 trans), 216.8 ((CO)5 cis), 149.3, 147.0 and the rest n.o. (C5, C4, C3, C2, C5′, C4′), n.o. (C7CH2, C6CH2 and C6′CH2), 15.1 (C7CH3), 15.0 (C6CH3), 15.8 (C6′CH3).
5: UV-Visλmax (CH2Cl2)/nm 600 (ε/dm3 mol−1 cm−1 6760), 488 (900), 373 (44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490). FT-IRνCO (hexane)/cm−1 2026m (A1(1)), 1955s (B1), 1942m (A1(2)), 1889m (B2). 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 7.71 (1 H, d, 3J5′,4′ 5.3, H5′), 7.20 (1 H, d, 3J4′,5′ 5.3, H4′), 4.54 (2 H, q, 3J 7.1, C7CH2), 4.50 (2 H, q, 3J 7.1, C6CH2), 1.71 (3 H, t, 3J 7.1, C7CH3), 1.61 (3 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 286.8 (C7carb), 282.7 (C6carb), 220.5 and 220.2 (COtrans), 212.5 (COcis), 169.6 (C5), 161.3 (C4), 145.8 and 128.8 (C3 and C2), 133.0 (C5′), 120.4 (C4′), 79.7 (C7CH2), 79.5 (C6CH2), 14.8 (C7CH3), 14.7 (C6CH3). HR-MSm/z (C16H12O6S2W, 548.23 g mol−1) calculated: 546.9507, found: 546.9580 (8%, [M − H]−), calculated: 518.9557, found: 518.9627 (16%, [M − H − CO]−), calculated: 462.9659, found: 462.9383 (8%, [M − H − 3CO]−).
490). FT-IRνCO (hexane)/cm−1 2026m (A1(1)), 1955s (B1), 1942m (A1(2)), 1889m (B2). 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 7.71 (1 H, d, 3J5′,4′ 5.3, H5′), 7.20 (1 H, d, 3J4′,5′ 5.3, H4′), 4.54 (2 H, q, 3J 7.1, C7CH2), 4.50 (2 H, q, 3J 7.1, C6CH2), 1.71 (3 H, t, 3J 7.1, C7CH3), 1.61 (3 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 286.8 (C7carb), 282.7 (C6carb), 220.5 and 220.2 (COtrans), 212.5 (COcis), 169.6 (C5), 161.3 (C4), 145.8 and 128.8 (C3 and C2), 133.0 (C5′), 120.4 (C4′), 79.7 (C7CH2), 79.5 (C6CH2), 14.8 (C7CH3), 14.7 (C6CH3). HR-MSm/z (C16H12O6S2W, 548.23 g mol−1) calculated: 546.9507, found: 546.9580 (8%, [M − H]−), calculated: 518.9557, found: 518.9627 (16%, [M − H − CO]−), calculated: 462.9659, found: 462.9383 (8%, [M − H − 3CO]−).
6: 6H 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 6Br. 1H NMRδ1H 6H (400.13 MHz; CDCl3; Me4Si) 7.56 (1 H, s, H4′), 4.50 (2 H, q, 3J 6.8, C7CH2), 4.48 (2 H, q, 3J 7.2, C6CH2), 1.68 (3 H, t, 3J 6.8, C7CH3), 1.62 (3 H, t, 3J 7.2, C6CH3), 2.82 (2 H, t, 3J 7.3, CH2CH2CH2CH3), 1.71 (2 H, m, CH2CH2CH2CH3), 1.44 (2 H, tq, 3J 7.3, CH2CH2CH2CH3), 0.97 (3 H, t, 3J 7.3, CH2CH2CH2CH3). δ1H 6Br (400.13 MHz; CDCl3; Me4Si) 4.51 (2 H, q, 3J 7.0, C7CH2), 4.48 (2 H, q, 3J 7.2, C6CH2), 1.69 (3 H, t, 3J 7.0, C7CH3), 1.61 (3 H, t, 3J 7.2, C6CH3), 2.82 (2 H, t, 3J 7.3, CH2CH2CH2CH3), 1.69 (2 H, m, CH2CH2CH2CH3), 1.44 (2 H, tq, 3J 7.3, CH2CH2CH2CH3), 0.97 (3 H, t, 3J 7.3, CH2CH2CH2CH3).
2 6Br. 1H NMRδ1H 6H (400.13 MHz; CDCl3; Me4Si) 7.56 (1 H, s, H4′), 4.50 (2 H, q, 3J 6.8, C7CH2), 4.48 (2 H, q, 3J 7.2, C6CH2), 1.68 (3 H, t, 3J 6.8, C7CH3), 1.62 (3 H, t, 3J 7.2, C6CH3), 2.82 (2 H, t, 3J 7.3, CH2CH2CH2CH3), 1.71 (2 H, m, CH2CH2CH2CH3), 1.44 (2 H, tq, 3J 7.3, CH2CH2CH2CH3), 0.97 (3 H, t, 3J 7.3, CH2CH2CH2CH3). δ1H 6Br (400.13 MHz; CDCl3; Me4Si) 4.51 (2 H, q, 3J 7.0, C7CH2), 4.48 (2 H, q, 3J 7.2, C6CH2), 1.69 (3 H, t, 3J 7.0, C7CH3), 1.61 (3 H, t, 3J 7.2, C6CH3), 2.82 (2 H, t, 3J 7.3, CH2CH2CH2CH3), 1.69 (2 H, m, CH2CH2CH2CH3), 1.44 (2 H, tq, 3J 7.3, CH2CH2CH2CH3), 0.97 (3 H, t, 3J 7.3, CH2CH2CH2CH3).
7: UV-Visλmax (CH2Cl2)/nm 600 (ε/dm3 mol−1 cm−1 6180), 443 (7020), 378 (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740). FT-IRνCO (hexane)/cm−1 M(CO)5: 2066m (A1(1)), 1983 and 1976vw (B1), 1940s (E and A1(2)), M(CO)4: 2024m (A1(1)), 1950s (B1), 1940s (A1(2)), 1884m (B2). νCO (DCM)/cm−1 M(CO)5: 2064m (A1(1)), 1972vw (B1), 1927s (E and A1(2)), M(CO)4: 2019m (A1(1)), 1938s (B1), 1927s (A1(2)), 1862m (B2). 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 4.54 (2 H, q, 3J 7.1, C7CH2), 4.47 (2 H, q, 3J 7.1, C6CH2), 5.06 (2 H, q, 3J 7.1, C6′CH2), 4.12 (2 H, q, 3J 6.9, C5′CH2), 2.49–2.33 (2 H, m, C4′CH2), 2.16 (2 H, q, 3J 7.3, C4′CH2), 1.69 (6 H, t, 3J 7.1, C7CH3 and C6′CH3), 1.59 (3 H, t, 3J 7.1, C6CH3), 1.44 (3 H, t, 3J 6.9, C5′CH3), 0.71 (6 H, t, 3J 7.1, C4′(CH3)2). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 287.5 (C7carb), 278.8 (C6carb), 341.9 (C6′carb), 221.7 and 219.2 (CO4 trans), 212.4 (CO4 cis), 200.9 (CO5 trans), 197.2 (CO5 cis), 163.6 (C5), 161.9 (C4), 119.0 and 114.9 (C3 and C2), 165.4 (C5′), 147.1 (C4′), 79.7 (C7CH2), 79.1 (C6CH2), 81.5 (C6′CH2), 70.7 (C4′CR3), 70.1 (C5′CH2), 26.8 (C4′(CH2)2, br), 14.8 (C7CH3 and C6CH3, br), 14.7 (C5′CH3 and C6′CH3, br), 9.1 (C4′(CH3)2, br). HR-MSm/z (C31H30O13S2W2, 1042.38 g mol−1) calculated: 1041.0069, found: 1041.0203 (14%, [M − H]−), calculated: 1013.0120, found: 1013.0165 (7%, [M − H − CO]−), calculated: 985.0170, found: 985.0019 (7%, [M − H − 2CO]−).
740). FT-IRνCO (hexane)/cm−1 M(CO)5: 2066m (A1(1)), 1983 and 1976vw (B1), 1940s (E and A1(2)), M(CO)4: 2024m (A1(1)), 1950s (B1), 1940s (A1(2)), 1884m (B2). νCO (DCM)/cm−1 M(CO)5: 2064m (A1(1)), 1972vw (B1), 1927s (E and A1(2)), M(CO)4: 2019m (A1(1)), 1938s (B1), 1927s (A1(2)), 1862m (B2). 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 4.54 (2 H, q, 3J 7.1, C7CH2), 4.47 (2 H, q, 3J 7.1, C6CH2), 5.06 (2 H, q, 3J 7.1, C6′CH2), 4.12 (2 H, q, 3J 6.9, C5′CH2), 2.49–2.33 (2 H, m, C4′CH2), 2.16 (2 H, q, 3J 7.3, C4′CH2), 1.69 (6 H, t, 3J 7.1, C7CH3 and C6′CH3), 1.59 (3 H, t, 3J 7.1, C6CH3), 1.44 (3 H, t, 3J 6.9, C5′CH3), 0.71 (6 H, t, 3J 7.1, C4′(CH3)2). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 287.5 (C7carb), 278.8 (C6carb), 341.9 (C6′carb), 221.7 and 219.2 (CO4 trans), 212.4 (CO4 cis), 200.9 (CO5 trans), 197.2 (CO5 cis), 163.6 (C5), 161.9 (C4), 119.0 and 114.9 (C3 and C2), 165.4 (C5′), 147.1 (C4′), 79.7 (C7CH2), 79.1 (C6CH2), 81.5 (C6′CH2), 70.7 (C4′CR3), 70.1 (C5′CH2), 26.8 (C4′(CH2)2, br), 14.8 (C7CH3 and C6CH3, br), 14.7 (C5′CH3 and C6′CH3, br), 9.1 (C4′(CH3)2, br). HR-MSm/z (C31H30O13S2W2, 1042.38 g mol−1) calculated: 1041.0069, found: 1041.0203 (14%, [M − H]−), calculated: 1013.0120, found: 1013.0165 (7%, [M − H − CO]−), calculated: 985.0170, found: 985.0019 (7%, [M − H − 2CO]−).
8: 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 4.56 (4 H, q, 3J 7.1, C7CH2), 4.48 (4 H, q, 3J 7.1, C6CH2), 1.71 (6 H, t, 3J 7.1, C7CH3), 1.60 (6 H, t, 3J 7.1, C6CH3).
9: 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 7.55 (1 H, d, 3J5′,4′ 4.5, H5′), 7.31 (1 H, d, 3J4′,5′ 4.5, H4′), 4.78 (2 H, q, 3J 7.0, C7CH2), 4.71 (2 H, q, 3J 7.2, C6CH2), 1.72 (3 H, t, 3J 7.0, C7CH3), 1.61 (3 H, t, 3J 7.2, C6CH3).
11: λmax (CH2Cl2)/nm 648, 446 and 387, (not quantitative). νCO (hexane)/cm−1 2015m (A1(1)), 1968s (B1), 1949m (A1(2)), 1901m (B2). δ1H (400.13 MHz; CDCl3; Me4Si) 4.77 (4 H, q, 3J 7.0, C7CH2), 4.70 (4 H, q, 3J 7.0, C6CH2), 1.71 (6 H, t, 3J 7.0, C7CH3), 1.61 (6 H, t, 3J 7.0, C6CH3). δ13C (75.468 MHz; CDCl3; Me4Si) 314.4 (C7carb), 308.0 (C6carb), 243.9 and 242.7 (COtrans), 227.6 (COcis), 162.4 (C5), 157.6 (C4), 137.3 and 132.5 (C3 and C2), 76.7 (C7CH2), n.o. (C6CH2), 15.0 and 15.0 (C7CH3 and C6CH3). m/z (C28H20O12S3Cr2, 748.64 g mol−1) calculated: 746.8849, found: 746.8924 (5%, [M − H]−), calculated: 718.8900, found: 718.8957 (28%, [M − H − CO]−), calculated: 690.8951, found: 690.9036 (26%, [M − H − 2CO]−).
12: 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 7.60 (1 H, d, 3J5′,4′ 5.2, H5′), 7.32 (1 H, d, 3J4′,5′ 5.2, H4′), 4.55 (2 H, q, 3J 7.1, C7CH2), 4.50 (2 H, q, 3J 7.1, C6CH2), 1.73 (3 H, t, 3J 7.1, C7CH3), 1.61 (3 H, t, 3J 7.1, C6CH3).
14: 1H NMRδ1H 14H (400.13 MHz; CDCl3; Me4Si) 7.48 (1 H, s, H4′), 4.55 (2 H, q, 3J 7.0, C7CH2), 4.49 (2 H, q, 3J 7.0, C6CH2), 1.74 (3 H, t, 3J 7.1, C7CH3), 1.61 (3 H, t, 3J 7.1, C6CH3), 2.88 (2 H, t, 3J 7.3, CH2CH2CH2CH3), 1.75 (2 H, m, CH2CH2CH2CH3), 1.44 (2 H, tq, 3J 7.3, CH2CH2CH2CH3), 0.97 (3 H, t, 3J 7.3, CH2CH2CH2CH3). δ1H 14Br (400.13 MHz; CDCl3; Me4Si) 4.55 (2 H, q, 3J 7.1, C7CH2), 4.48 (2 H, q, 3J 7.1, C6CH2), 1.74 (3 H, t, 3J 7.1, C7CH3), 1.60 (3 H, t, 3J 7.1, C6CH3), 2.88 (2 H, t, 3J 7.2, CH2CH2CH2CH3), 1.74 (2 H, m, CH2CH2CH2CH3), 1.44 (2 H, tq, 3J 7.2, CH2CH2CH2CH3), 0.97 (3 H, t, 3J 7.2, CH2CH2CH2CH3).
15: 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 8.74 (1 H, s, H4′), 4.51 (2 H, q, 3J 7.1, C7CH2), 4.48 (2 H, q, 3J 7.2, C6CH2), 5.17 (2 H, q, 3J 6.7, C6′CH2), 1.74 (3 H, t, 3J 7.1, C7CH3), 1.63 (3 H, t, 3J 7.2, C6CH3), 1.76 (3 H, t, 3J 7.1, C6′CH3).
16: UV-Visλmax (CH2Cl2)/nm 630 (ε/dm3 mol−1 cm−1 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590), 456 (38
590), 456 (38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240), 379 (46
240), 379 (46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210). FT-IRνCO (hexane)/cm−1 2023m (A1(1)), 1961s (B1), 1946m (A1(2)), 1894m (B2). 1H NMRδ1H (300.13 MHz; CDCl3; Me4Si) 4.54 (4 H, q, 3J 7.1, C7CH2), 4.47 (4 H, q, 3J 7.1, C6CH2), 1.72 (6 H, t, 3J 7.1, C7CH3), 1.62 (6 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 286.0 (C7carb), 280.7 (C6carb), 220.4 and 219.7 (COtrans), 212.0 (COcis), 169.0 (C5), 162.1 (C4), 137.4 and 133.8 (C3 and C2), 79.7 (C7CH2), 79.6 (C6CH2), 14.7 (C7CH3 and C6CH3). HR-MSm/z (C28H20O12S3W2, 1012.33 g mol−1) calculated: 1010.9058, found: 1010.9023 (100%, [M − H]−), calculated: 982.9109, found: 982.9065 (16%, [M − H − CO]−), calculated: 954.9159, found: 954.9031 (6%, [M − H − 2CO]−).
210). FT-IRνCO (hexane)/cm−1 2023m (A1(1)), 1961s (B1), 1946m (A1(2)), 1894m (B2). 1H NMRδ1H (300.13 MHz; CDCl3; Me4Si) 4.54 (4 H, q, 3J 7.1, C7CH2), 4.47 (4 H, q, 3J 7.1, C6CH2), 1.72 (6 H, t, 3J 7.1, C7CH3), 1.62 (6 H, t, 3J 7.1, C6CH3). 13C NMRδ13C (100.613 MHz; CDCl3; Me4Si) 286.0 (C7carb), 280.7 (C6carb), 220.4 and 219.7 (COtrans), 212.0 (COcis), 169.0 (C5), 162.1 (C4), 137.4 and 133.8 (C3 and C2), 79.7 (C7CH2), 79.6 (C6CH2), 14.7 (C7CH3 and C6CH3). HR-MSm/z (C28H20O12S3W2, 1012.33 g mol−1) calculated: 1010.9058, found: 1010.9023 (100%, [M − H]−), calculated: 982.9109, found: 982.9065 (16%, [M − H − CO]−), calculated: 954.9159, found: 954.9031 (6%, [M − H − 2CO]−).
17: 1H NMRδ1H (400.13 MHz; CDCl3; Me4Si) 4.62 (4 H, dq, 3J 7.1, C7CH2), 4.50 (4 H, dq, 3J 7.2, C6CH2), 1.63 (6 H, t, 3J 7.1, C7CH3), 1.53 (6 H, t, 3J 7.2, C6CH3). 13C NMRδ13C (75.468 MHz; CDCl3; Me4Si) 296.5 (C7carb), 282.9 (C6carb), 219.4 and 216.8 (COtrans), 212.4 and 210.7 (COcis), 169.0 (C5), 162.1 (C4), 150.3 and 129.0 (C3 and C2), 80.3 (C7CH2), 79.5 (C6CH2), 14.8 and 14.7 (C7CH3), 14.9 and 14.9 (C6CH3).
Characterization and analytical techniques
Compounds 1 and 2 did not ionize during MS analysis, and therefore the data are neglected. Only proton NMR analysis was carried out on 6, 8, 9, 12, 14 and 15 as the compounds were obtained as an inseparable mixture or were too reactive for further analysis. The reactivity of 4 and 17 allowed only proton and carbon NMR analysis to be obtained, before decomposing.First-order analysis is carried out to assign signals of the 1H NMR spectra. Additional 2D [1H, 1H] COSY NMR experiments were done where confirmation of the proton assignments was required. Assigning the carbon chemical shifts, obtained from proton-decoupled 13C NMR spectra, was possible with the assistance of 2D [1H, 13C] HSQC and 2D [1H, 13C] HMBC NMR experiments (see ESI section S3†). Standard Bruker pulse programs were used in the experiments.
UV-Vis spectroscopy
Measurements were performed on 10 mL DCM solutions of 0.01 mM analyte concentration at 25 °C. Absorptions were measured in the range 200–1000 nm using a UV-Vis spectrophotometer Specord 200 plus. WinASPECT PLUS (version 4.2) software was used for data visualization.X-Ray diffraction analysis
Single crystal diffraction data for 1, 3, 5, 7, 11 and 16, were collected at 150 K on a Bruker D8 Venture diffractometer with a kappa geometry goniometer and a Photon 100 CMOS detector using a Mo-Kα IμS.micro focus source. Data were reduced and scaled using SAINT and absorption intensity corrections were performed using SADABS (APEX III control software).52 A single crystal of 2 was analyzed on a Rigaku XtaLAB Synergy R diffractometer, with a rotating-anode X-ray source and a HyPix CCD detector. Data reduction and absorption were carried out using the CrysAlisPro (version 1.171.40.23a) software package.53 X-ray diffraction measurements were performed at 293 K using an Oxford Cryogenics Cryostat. All structures were solved by an intrinsic phasing algorithm using SHELXTS54 and were refined by full-matrix least-squares methods based on F2 using SHELXL.55 All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were placed in idealized positions and refined using riding models. The crystal data collection and structure refinement parameters are provided in the ESI.†Cyclic voltammetry
The cyclic voltammograms were recorded in dry, oxygen-free, high performance liquid chromatography (HPLC) grade DCM using a three-electrode cell. The three electrodes are; the non-aqueous Ag/Ag+ reference electrode, a glassy carbon working electrode and the platinum wire auxiliary (counter) electrode. Measurements were carried out on a Metrohm μAutolab type III potentiostat, using NOVA 2.0 electrochemistry software. Measurements were done on 1.0 mM solutions of the compounds that contain 0.1 M [NnBu4][PF6] as supporting electrolyte, at an scan rates of 100 mV s−1 at 20 °C. FcH or [Fe(η5-C5Me5)2] were used as internal standards (1.0 mM) vs. the Ag/Ag+ couple, with E°′ = 0 V for the FcH0/+1 couple and E°′ = −0.6 V for the [Fe(η-C5Me5)2]0/+1 couple. Compounds 16, 18, 20 and 21 (with FcH as internal standard) and 1, 3, 11 and 19 (with [Fe(η5-C5Me5)2] as internal standard) were studied by cyclic voltammetry.Computational details
Geometry optimizations of all species discussed in the text were carried out with the GAUSSIAN 09 suite of programs.56 Electron correlation was partially taken into account using the B3LYP57–59 functional in conjuction with the D3 dispersion correction suggested by Grimme et al.60 and the double-ζ quality plus polarisation functions def2-SVP61 basis set for all atoms. This level is denoted (u)B3LYP-D3/def2-SVP. All species were characterized by frequency calculations,62 and have positive definite Hessian matrices. Cartesian coordinates (in Å) and total energies (in a. u., ZPVE included) of all the stationary points are available in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the National Research Foundation, South Africa (NRF 105740; NRF 105529, NRF 87788 and NRF 9772) and Sasol Technology R&D Pty. Ltd. (South Africa) for financial support. I. F. acknowledges support from the Spanish MINECO (CTQ2016-78205-P, CTQ2016-81797-REDC and PID2019-106184GB-I00).References
- A. Rajca, M. Miyasaka, M. Pink, H. Wang and S. Rajca, J. Am. Chem. Soc., 2004, 126, 15211 CrossRef CAS.
- G. M. Tsivgoulis and J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1995, 34, 1119 CrossRef CAS.
- Molecular Switches, ed. B. L. Feringa, Wiley-VCH, Weinheim, 2001 Search PubMed.
- M.-C. Chen, Y.-J. Chiang, C. Kim, Y.-J. Guo, S.-Y. Chen, Y.-J. Liang, Y.-W. Huang, T.-S. Hu, G.-H. Lee, A. Facchetti and T. J. Marks, Chem. Commun., 2009, 1846 RSC.
- N. J. Long, Angew. Chem., Int. Ed. Engl., 1995, 34, 21 CrossRef CAS.
- I. S. Lee, H. Seo and Y. K. Chung, Organometallics, 1999, 18, 5194 CrossRef.
- C. Q. Ma, Front. Optoelectron., 2011, 4, 12 CrossRef.
- W. Jiang, Y. Li and Z. Wang, Chem. Soc. Rev., 2013, 42, 6113 RSC.
- S. S. Bukalov, L. A. Leites, K. A. Lyssenko, R. R. Aysin, A. A. Korlyukov, J. V. Zubavichus, K. Y. Chernichenko, E. S. Balenkova, V. G. Nenajdenko and M. Y. Antipin, J. Phys. Chem. A, 2008, 112, 10949 CrossRef CAS.
- V. G. Nenajdenko, D. V. Gribkov, V. V. Sumerin and E. S. Balenkova, Synthesis, 2003, 124 CrossRef CAS.
- T. Ozturk, E. Ertas and O. Mert, Tetrahedron, 2005, 61, 11055 CrossRef CAS.
- K. Y. Chernichenko, E. S. Balenkova and V. G. Nenajdenko, Mendeleev Commun., 2008, 18, 171 CrossRef CAS.
- S. Roué, H. Sahoune, L. Toupet, J.-F. Halet and C. Lapinte, Organometallics, 2016, 35, 2057 CrossRef.
- Y. Tanaka, T. Ishisaka, A. Inagaki, T. Koike, C. Lapinte and M. Akita, Chem. – Eur. J., 2010, 16, 4762 CrossRef CAS.
- J. Barluenga, M. A. Fernández-Rodríguez, E. Aguilar, F. Fernández-Marí, A. Salinas and B. Olano, Chem. – Eur. J., 2001, 7, 3533 CrossRef CAS.
- R. Stumpf, M. Jaeger and H. Fischer, Organometallics, 2001, 20, 4040 CrossRef CAS.
- T. S. Powers, W. D. Wulff, J. Quinn, Y. Shi, W. Jiang, R. Hsung, M. Parisi, A. Rahm, X. Wu Jiang, G. P. A. Yap and A. L. Rheingold, J. Organomet. Chem., 2001, 617–618, 182 CrossRef CAS.
- M. Jaeger, R. Stumpf, C. Troll and H. Fischer, Chem. Commun., 2000, 1, 931 RSC.
- D. B. Grotjahn, F. E. K. Kroll, T. Schäfer, K. Harms and K. H. Dötz, Organometallics, 1992, 11, 298 CrossRef CAS.
- M. A. Sierra, Chem. Rev., 2000, 100, 3591 CrossRef CAS.
- M. Landman, R. Pretorius, B. E. Buitendach, P. H. van Rooyen and J. Conradie, Organometallics, 2013, 32, 5491 CrossRef CAS.
- H. G. Raubenheimer, S. Cronje and C. E. Strasser, Dalton Trans., 2009, 8145 RSC.
- R. L. Beddoes, J. E. Painter, P. Quayle and P. Patel, Tetrahedron, 1997, 53, 17297 CrossRef CAS.
- N.-A. Weststrate, S. Bouwer, C. Hassenrück, N. A. van Jaarsveld, D. C. Liles, R. F. Winter and S. Lotz, J. Organomet. Chem., 2018, 869, 54 CrossRef CAS.
- N. A. van Jaarsveld, D. C. Liles and S. Lotz, Dalton Trans., 2010, 39, 5777 RSC.
- N. Hoa Tran Huy, P. Lefloch, J. M. Louis and M. Fetizon, J. Organomet. Chem., 1986, 311, 79 CrossRef.
- N. Hoa Tran Huy, E. O. Fischer, J. Riede, U. Thewalt and K. H. Dötz, J. Organomet. Chem., 1984, 263, C29–C32 CrossRef.
- N. Hoa Tran Huy, E. O. Fischer, H. G. Alt and K. H. Dötz, J. Organomet. Chem., 1985, 284, C9–C11 CrossRef CAS.
- E. O. Fischer, W. Röll, N. Hoa Tran Huy and K. Ackermann, Chem. Ber., 1982, 115, 2951 CrossRef CAS.
- K. Öfele, F. Dyckhoff and H. G. Krist, Chem. Ber., 1993, 126, 2573 CrossRef.
- N. Hoa Tran Huy and E. O. Fischer, Nouv. J. Chim., 1985, 9, 257 Search PubMed.
- Z. Lamprecht, N. A. van Jaarsveld, D. I. Bezuidenhout, D. C. Liles and S. Lotz, Dalton Trans., 2015, 44, 19218 RSC.
- F. W. Evans, R. J. Fox and M. Szwarc, J. Am. Chem. Soc., 1960, 82, 6414 CrossRef CAS.
- M. J. Diem, D. F. Burow and J. L. Fry, J. Org. Chem., 1977, 42, 1801 CrossRef CAS.
- M. Landman, H. Görls and S. Lotz, J. Organomet. Chem., 2001, 617–618, 280 CrossRef CAS.
- Z. Lamprecht, S. G. Radhakrishnan, A. Hildebrandt, H. Lang, D. C. Liles, N.-A. Weststrate, S. Lotz and D. I. Bezuidenhout, Dalton Trans., 2017, 46, 13983 RSC.
- D. M. Adams, Metal-Ligand and Related Vibrations, Edward Arnold Publishers Ltd, London, 1967 Search PubMed.
- D. M. Andrada, M. E. Zoloff Michoff, I. Fernández, A. M. Granados and M. A. Sierra, Organometallics, 2007, 26, 5854 CrossRef CAS.
- I. Fernández, F. P. Cossio, A. Arrieta, B. Lecea, M. J. Mancheño and M. A. Sierra, Organometallics, 2006, 23, 1065 CrossRef.
- N. Lugan, I. Fernández, R. Brousses, D. A. Valyaev, G. Lavigne and N. A. Ustynyuk, Dalton Trans., 2013, 42, 898 RSC.
- D. A. Valyaev, R. Brousses, N. Lugan, I. Fernández and M. A. Sierra, Chem. – Eur. J., 2011, 17, 6602 CrossRef CAS.
- B. van der Westhuizen, P. J. Swarts, I. Strydom, D. C. Liles, I. Fernández, J. C. Swarts and D. I. Bezuidenhout, Dalton Trans., 2013, 42, 5367 RSC.
- M. Guricová, T. Tobrman, M. Pižl, S. Žižková, I. Hoskovcová and D. Dvořák, J. Organomet. Chem., 2020, 905, 121023 CrossRef.
- D. I. Bezuidenhout, B. van der Westhuizen, I. Strydom, P. J. Swarts, J. C. Swarts and I. Fernández, Inorg. Chim. Acta, 2014, 423, 184 CrossRef CAS.
- G. M. Chu, I. Fernández and M. A. Sierra, Chem. – Eur. J., 2013, 19, 5899 CrossRef CAS.
- H. Meerwein, Org. Synth., 1966, 46, 113 CrossRef CAS.
- V. G. Nenajdenko, D. V. Gribkov, V. V. Sumerin and E. S. Balankova, Synthesis, 2003, 1, 124 CrossRef.
- F. Allared, J. Hellberg and T. Remonen, Tetrahedron Lett., 2002, 43, 1553 CrossRef CAS.
- L. Brandsma, S. F. Vasilevsky and H. D. Verkruijsse, Application of transition metal catalysts in organic synthesis, Springer, 1998 Search PubMed.
- L. Yang, P. Ye, W. Li, W. Zhang, Q. Guan, C. Ye, T. Dong, X. Wu, W. Zhao, X. Gu, Q. Peng, B. Tang and H. Huang, Adv. Opt. Mater., 2018, 6, 1 Search PubMed.
- Z. Lamprecht, F. P. Malan, D. C. Liles, S. Lotz and D. I. Bezuidenhout, J. Organomet. Chem., 2020, 925, 121466 CrossRef CAS.
- APEX3. (including SAINT and SADABS), BrukerAXS Inc., Madison, WI, 2016 Search PubMed.
- Rigaku Oxford Diffraction, Rigaku Corporation, Oxford, UK, 2018 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, et al., Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- J. W. McIver and A. Komornicki, J. Am. Chem. Soc., 1972, 94, 2625 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis details, NMR and FT-IR spectra, crystal data collection and crystal packing details and cartesian coordinates for optimized geometries. CCDC 2032754–2032759. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt03298k |
| This journal is © The Royal Society of Chemistry 2020 |