A green separation process of Ag via a Ti4(embonate)6 cage†
Abstract
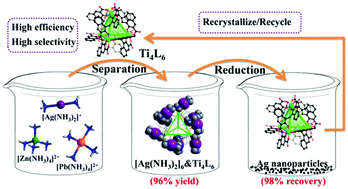
From an environmental perspective, silver recovery through a green process is imperative. In this work, a green supramolecular separation process of Ag has been developed by using a highly charged anionic Ti4L6 (L = embonate) cage as the extractant. Such a Ti4L6 cage has unique selectivity toward [Ag(NH3)2]+ ions, because only linear [Ag(NH3)2]+ ions can be trapped into the windows of the Ti4L6 cage, which is demonstrated by single-crystal X-ray diffraction analysis. To further illustrate the efficiency and mechanism of the herein constructed silver separation method, three co-crystals of the Ti4L6 cage with various [Ag(NH3)2]+ ions were prepared and structurally characterized, annotating the stepwise recognition of [Ag(NH3)2]+ ions by the Ti4L6 extractant. However, it failed to trap larger tetrahedral [Zn(NH3)4]2+ and quadrilateral [Pb(NH3)4]2+ ions under the same reaction conditions, indicating that configuration matching contributes to the high selectivity of the above-mentioned silver separation procedure. More interestingly, Ag nanoparticles with high yield could be obtained by the reduction of the [Ag(NH3)2]&Ti4L6 extracts with hydrazine hydrate (N2H4·H2O), and the Ti4L6 cages can be readily recycled through recrystallization. This discovery offers a green supramolecular procedure for silver recovery with coordination cages as efficient and recyclable extractants.
- This article is part of the themed collections: Spotlight Collection: Metallocycles and Metallocages and Inorganic chemistry approaches to saving critical elements: Recovery, Reuse and Recycling


 Please wait while we load your content...
Please wait while we load your content...