Neatly arranged mesoporous MnO2 nanotubes with oxygen vacancies for electrochemical energy storage†
Abstract
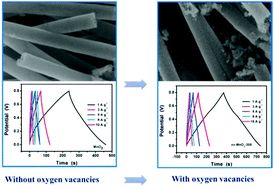
Intrinsically poor conductivity, sluggish ion transfer kinetics, and limited specific area are the three main obstacles that confine the electrochemical performance of manganese dioxide in supercapacitors. Herein, one-dimensional mesoporous MnO2 nanotubes were prepared using a polycarbonate film as a template and a large number of oxygen vacancies were introduced by calcination under a N2 atmosphere. The effects of calcination temperature on the crystal structure, micro-morphology and electrochemical performance of MnO2 nanotubes were studied. The presence of oxygen vacancies increases the redox capacity of ov-MnO2-300 nanotubes, and the unique one-dimensional mesoporous structure also provides an effective channel for ion transport. Therefore, the ov-MnO2-300 nanotube has an excellent specific capacitance of 459.0 F g−1 at a current density of 1 A g−1 and also has outstanding rate performance and cycle performance. An asymmetric supercapacitor assembled with ov-MnO2-300 nanotubes as the positive electrode and graphene@MoS2 as the negative electrode delivered an energy density of 40.2 W h kg−1 at a power density of 1024 W kg−1. The excellent capacitance performance is mostly attributed to the introduction of oxygen vacancies to increase the intrinsic conductivity of MnO2, and the unique one-dimensional mesoporous nanotube structure increases the active sites of redox reactions.


 Please wait while we load your content...
Please wait while we load your content...