Photoinduced synergistic cytotoxicity towards cancer cells via Ru(ii) complexes†
Abstract
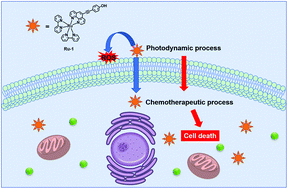
Ru(II) complexes have been recognized as excellent anticancer candidates. However, the poor cell uptake of these complexes has been the major obstacle in improving their anticancer efficacy. Along with the development of the knowledge on the anticancer nature of the Ru(II) complexes, several strategies have been designed to increase the cellular accumulation of these complexes. Among them, the light-triggered drug uptake approach is most promising. In this study, a bioactive Ru(II)–polypyridyl complex Ru-1 is constructed via incorporating a modified phenanthroline ligand into the enlarged conjugate structure. The modulation of the ligand makes Ru-1 powerful in generating singlet oxygen and effective in photodynamic therapeutic activity. Moreover, the cell uptake is improved during this photodynamic process and induces further chemotherapeutic effect, which act synergistically to enhance its anticancer activity. The photoinduced synergistic cytotoxicity of Ru-1 towards cancer cells offers an effective way to sensitize the antitumor activity of Ru(II) complexes.


 Please wait while we load your content...
Please wait while we load your content...