Fabrication of 2D/2D nanosheet heterostructures of ZIF-derived Co3S4 and g-C3N4 for asymmetric supercapacitors with superior cycling stability†
Abstract
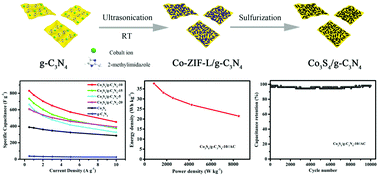
Metal sulfides with high activity are favorable electrode materials for supercapacitors. However, their relatively inferior electronic conductivity and poor stability in alkaline electrolyte solutions impede their applications. To overcome these drawbacks, herein, 2D/2D nanosheet heterostructures of Co3S4 and g-C3N4 have been successfully fabricated by a facile method that involves the in situ growth of 2D Co-based zeolitic imidazolate framework (Co-ZIF-L) crystals on g-C3N4 nanosheets followed by subsequent sulfurization. The as-prepared Co3S4/g-C3N4-10 exhibits a largely enhanced specific capacity (415.0 C g−1 at 0.5 A g−1) in comparison with solitary g-C3N4 (18.9 C g−1) and Co3S4 (194.4 C g−1) derived from Co-ZIF-L. Furthermore, it also displays good rate capability (54.5% retention at 10 A g−1). The asymmetric supercapacitor fabricated from Co3S4/g-C3N4-10 and activated carbon electrodes exhibits an outstanding energy density of 35.7 W h kg−1 at a high power density of 850.2 W kg−1. Most importantly, the asymmetric supercapacitor demonstrates an ultrahigh cycling durability with only 1.9% capacitance loss after 10 000 cycles at 10 A g−1. This superior electrochemical performance can be attributed to the unique 2D/2D nanosheet heterostructures providing rich active sites, short ion diffusion pathways, fast charge transfer as well as improved conductivity and mechanic stability. This work may pave the way for a rational design of the heterostructures of metal sulfides and g-C3N4 for electrochemical energy storage devices with a long cycling lifespan.


 Please wait while we load your content...
Please wait while we load your content...