Visible and near-infrared driven Yb3+/Tm3+ co-doped InVO4 nanosheets for highly efficient photocatalytic applications†
Abstract
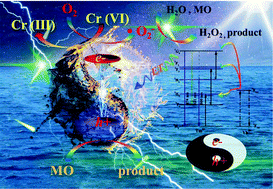
To effectively enhance the utilization of clean sunlight energy, harvesting a large percentage of near infrared (NIR) light is significant. One of the commonly used effective methods for modifying semiconductors is by co-doping upconversion materials on semiconductors to heighten the photocatalytic efficiency. In this work, Yb3+/Tm3+ co-doped InVO4 nanosheets were synthesized by a facile hydrothermal path, and the crystal phases, morphologies, surface chemical compositions, as well as optical properties were characterized. Yb3+/Tm3+ co-doped InVO4 revealed significantly enhanced photoactivity towards chromium(VI) reduction and methyl orange oxidation under visible or NIR light irradiation. Furthermore, 5YT-IV presented the highest electrocatalytic performance and photocatalytic production of H2O2 under visible light irradiation, requiring low overpotential and low Tafel slope (390 mV dec−1) for hydrogen evolution reaction than that of the bare InVO4 (731 mV dec−1), and as well improved the yield of photocatalytic H2O2 production by about 3.5 times. This was primarily ascribed to intensive light absorption resulting from the benign upconversion energy transfer of Yb3+/Tm3+ and the boosted charge separation caused by the intermediate energy states. Moreover, the presence of h+ and ˙O2− as the main oxidative species played a significant role during the photocatalytic oxidation process of methyl orange and electrons played a decisive role in Cr(VI) reduction. This study provides a promising platform for efficiently utilizing the visible-NIR energy of sunlight in the field of photocatalytic H2O2 production and for alleviating environmental pollution in future.


 Please wait while we load your content...
Please wait while we load your content...