Open Access Article
Open Access ArticleINSIGHTS into the structures adopted by titanocalix[6 and 8]arenes and their use in the ring opening polymerization of cyclic esters†
Orlando
Santoro
a,
Mark R. J.
Elsegood
 *b,
Elizabeth V.
Bedwell
b,
Jake A.
Pryce
b and
Carl
Redshaw
*b,
Elizabeth V.
Bedwell
b,
Jake A.
Pryce
b and
Carl
Redshaw
 *a
*a
aPlastics Collaboratory, Department of Chemistry and Biochemistry, The University of Hull, Cottingham Road, Hull, HU6 7RX, UK. E-mail: C.Redshaw@hull.ac.uk
bChemistry Department, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK
First published on 10th August 2020
Abstract
Interaction of p-tert-butylcalix[6]areneH6, L1H6, with [TiCl4] afforded the complex [Ti2Cl3(MeCN)2(OH2)(L1H)][Ti2Cl3(MeCN)3(L1H)]·4.5MeCN (1·4.5MeCN), in which two pseudo-octahedral titanium centres are bound to one calix[6]arene. A similar reaction but employing THF resulted in the THF ring-opened product [Ti4Cl2(μ3-O)2(NCMe)2(L)2(O(CH2)4Cl)2]·4MeCN (2·4MeCN), where LH4 = p-tert-butylcalix[4]areneH4. Interaction of L1H6 with [TiF4] (3 equiv.) led, after work-up, to the complex [(TiF)2(μ-F)L1H]2·6.5MeCN (3·6.5MeCN). Treatment of p-tert-butylcalix[8]areneH8, L2H8, with [TiCl4] led to the isolation of the complex [(TiCl)2(TiClNCMe)2(μ3-O)2(L2)]·1.5MeCN (4·1.5MeCN). From a similar reaction, a co-crystallized complex [Ti4O2Cl4(MeCN)2(L2)][Ti3Cl6(MeCN)5(OH2)(L2H2)]·H2O·11MeCN (5·H2O 11MeCN) was isolated. Extension of the L2H8 chemistry to [TiBr4] afforded, depending on the stoichiometry, the complexes [(TiBr)2(TiBrNCMe)2(μ3-O)2(L2)]·6MeCN (6·6MeCN) or [[Ti(NCMe)2Br]2[Ti(O)Br2(NCMe)](L2)]·7.5MeCN (7·7.5MeCN), whilst use of [TiF4] afforded complexes containing Ca2+ and Na+, thought to originate from drying agents, namely [Ti8CaF20(OH2)Na2(MeCN)4(L2)2]·14MeCN (8·14MeCN), [Na(MeCN)2][Ti8CaF20NaO16(L2)2]·7MeCN (9·7MeCN) or [Na]6[Ti8F20Na(MeCN)2(L2)][Ti8F20Na(MeCN)0.5(L2)]·15.5(C2H3N) (10·15.5MeCN). In the case of [TiI4], the ladder [(TiI)2(TiINCMe)2(μ3-O)2(L2)]·7.25CH2Cl2 (11·7.25CH2Cl2) was isolated. These complexes have been screened for their potential to act as catalysts in the ring opening polymerization (ROP) of ε-caprolactone (ε-CL), δ-valerolactone (δ-VL) and rac-lactide (r-LA), both in air and N2. For ε-CL and δ-VL, moderate activity at 130 °C over 24 h was observed for 1, 9 and 11; for r-LA, only 1 exhibited reasonable activity. In the case of the co-polymerization of ε-CL with δ-VL, the complexes 1 and 11 afforded reasonable conversions and low molecular weight polymers, whilst 4, 6, and 9 were less effective. None of the complexes proved to be active in the co-polymerization of ε-CL and r-LA under the conditions employed herein.
Introduction
Frameworks capable of binding multiple metal centres are of interest in catalysis given the potential for beneficial cooperative effects.1 Our interest in this area has been, and remains, focused mostly around the use of the family of polyphenolic macrocycles called calix[n]arenes.2 For the n = 4 system, namely p-tert-butylcalix[4]areneH4 (LH4), the tendency is to coordinate to only one metal centre via the four phenolic oxygens (the lower rim) and usually the macrocycle retains the cone conformation.3 Of the larger calix[n]arenes, the n = 6 (L1H6) and 8 (L2H8) systems are attractive scaffolds given their availability; odd numbered calix[n]arenes are isolated in far lower yields.4 However, the coordination chemistry of the larger calix[n]arenes remains relatively unexplored.2,5 In the case of titanium, reports date back to the 1980s.6 In our coordination studies employing different metals (i.e. tungsten and vanadium), we have had limited success for n = 6,7 whilst previous work for n = 8 has shown that it is possible, via controlling the reaction stoichiometry, to incorporate selectively two, three, or four metal centres (W) at the lower rim.8 Furthermore, the systems incorporating vanadium have exhibited high catalytic activities in the area of α-olefin polymerization.9 In the area of ring opening polymerization (ROP) of cyclic esters, reports using metallocalix[n]arenes are scant. In the case of tungstocalix[6 and 8]arenes, we observed how different sized calixarene rings and their associated conformations can drastically affect the catalytic activity for the ROP of ε-caprolactone (ε-CL).10 More recently, McIntosh et al. reported preliminary studies on the use of the complex [Ti4(L2)(On-Pr)8(THF)2] as a catalyst for the ROP of rac-lactide (r-LA) at 130 °C.11 Other titanocalix[n]arene work in this area employs the de-tert-butylated n = 4 system (1,3-di-n-propylcalix[4]arene), with well-behaved ROP of rac-lactide observed when employing either microwave radiation or heat; the former method was beneficial to the rate of polymerization at the expense of control.12 A related system, possessing para-NO2 and tert-butyl groups at the upper-rim of the calix[4]arene was capable of the well-controlled ROP of L- and r-LA under solvent-free conditions.13 Recently, we have tested the efficiency of known complexes of the type [TiCl2L(O)2(OR)2] (R = Me, n-Pr and n-pentyl), the Cl-bridged compound {[TiL(O)3(OR)]2(μ-Cl)2} (R = n-decyl) and the monochloride complex [Ti(NCMe)ClL(O)3(OMe)] in the ROP of several cyclic esters.14 Although all complexes were found to be efficient for ROP, the monochloride species proved to be the best performing of the series. It is noteworthy that all catalysts were shown to be active even under aerobic conditions, without any significant activity loss. Moreover, titanocalix[4]arene species were shown to be better performing than other Ti-based benchmark catalysts (including a Ti-diphenolate compound), suggesting a positive effect of the calix[4]arene ligand on the catalyst efficiency. These limited studies suggest there is the potential for accessing both controllable and highly active ROP catalysts based on titanocalix[n]arenes with n ≥ 6. Herein, we focus on titanocalix[6 and 8]arenes derived from interaction of the parent p-tert-butylcalix[6 and 8]arenes, namely n = 6 (L1H6) and n = 8 (L2H8) with the tetrahalides [TiX4] (X = Cl, Br, F, I). A number of intriguing molecular structures (see Chart 1 and Fig. S1 and 2, ESI†) have been identified, and the complexes (not 2, 5, 7, and 8) have been screened for their ability to act as catalysts in the ROP of ε-CL, δ-valerolactone (δ-VL), and r-LA as well as for the copolymerization of ε-CL with δ-VL, and ε-CL with r-LA. We have recently reviewed the use of titanium diphenolates and titanocalix[4]arenes for both α-olefin polymerization and the ROP of cyclic esters.15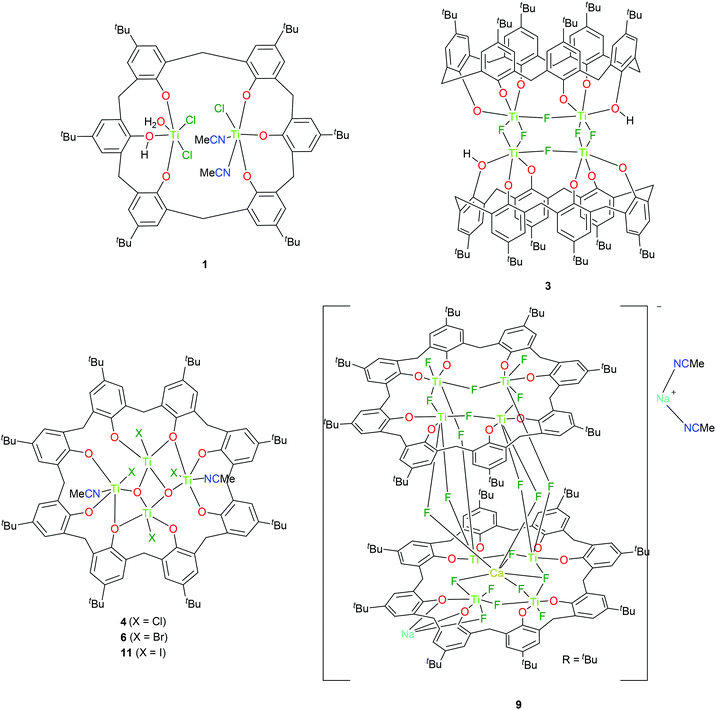 | ||
| Chart 1 Titanocalix[6 and 8]arene complexes 1, 3, 4, 6, 9 and 11 prepared herein and tested as catalysts for the ROP of cyclic esters. (Complexes 2, 5, 7, and 8 were not tested). | ||
Results and discussion
Use of p-tert-butylcalix[6]areneH6 L1H6
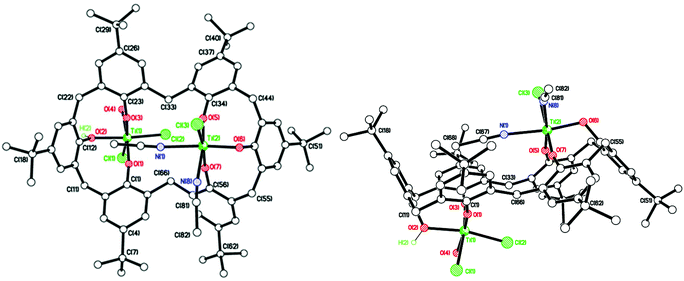
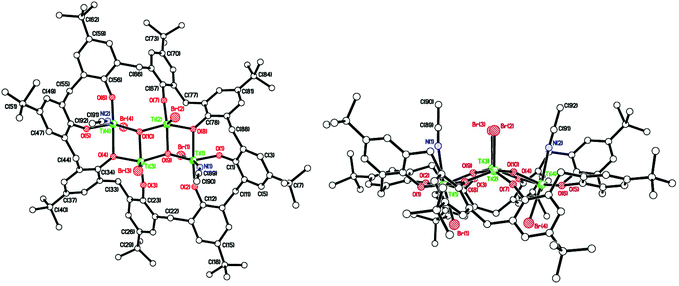
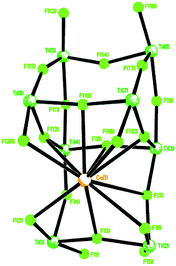
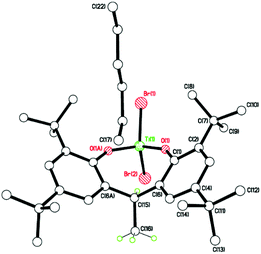
In the case of L1H6, where L1 = p-tert-butylcalix[6]arene, interaction with two equivalents of [TiCl4] afforded, after work-up (MeCN), the complex [Ti2Cl3(MeCN)2(OH2)(L1H)] [Ti2Cl3(MeCN)3(L1H)]·4.5 MeCN (1·4.5 MeCN) as orange crystals on slow cooling to ambient temperature. In the IR spectrum, v(CN) for both coordinated and free acetonitrile (2319/2308 and 2289 cm−1, respectively) are observed. The molecular structure (CCDC 1973136†) of 1 is shown in Fig. 1, with selected bond lengths and angles given in the caption. The asymmetric unit contains two similar but unique molecules. In each case, the two pseudo-octahedral Ti(IV) ions are bound to an L1H ligand via three phenolate oxygens to each titanium ion; the coordinated chlorides, acetonitrile molecules and water molecule are facial. In each molecule; the phenolic hydrogens on O(2) and O(2A) are retained, whilst Ti(1) is bonded to two Cl− ions and one water molecule, Ti(1A) is bonded to two Cl− ions and one MeCN molecule, and this is the chemical difference between the two unique metal complexes; Ti(2) and Ti(2A) are both bonded to one Cl− ion and two MeCN molecules. There are hydrogen bonds between O(2)–H(2)⋯O(6A) and O(2A)–H(2A)⋯O(6). The retention of a phenolic hydrogen on L1, allows for hydrogen bonding with an oxygen on the other molecule in the asymmetric unit, or the next pair along the chain. These pairs of molecules form infinite, H-bonded, zig-zag chains, in the b-direction (see Fig. S3, ESI†). The coordination of the metal to the calix[6]arene and the conformation adopted by the macrocycle are reminiscent of that observed for the group V complexes {[M(NCMe)Cl2]2L1} (M = Nb, Ta).16 When THF was employed as solvent, the orange/red complex [Ti4Cl2(μ3-O)2(NCMe)2(L)2(O(CH2)4Cl)2]·4MeCN (2·4MeCN) was isolated in low yield. Its molecular structure (CCDC 1973134†) is shown in Fig. S4 of the ESI.† The molecule lies on a centre of symmetry, and was refined as a 2-component twin (components 0.5431![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4569(10)); component 2 rotated by 8.1802° around [−0.01 0.98 0.18] (reciprocal) or [0.20 0.92 0.33] (direct). The core of the complex can be described as two, singly vertex–vacant cubes, connected by a face, in which the Ti octahedra share edges. Both Ti(2) and Ti(2A) possess O(CH2)4Cl groups. The main core of 2 was found to be similar to that of [Ti(NCMe)(μ3-O)L(O)4TiCl(O(CH2)4Cl)]2–2[TiCl(NCMe)(L(O)3(On-Pr))]·11MeCN, a compound we have recently reported.14 The formation of 2 is thought to involve the ring opening of the THF, which has been reported in the literature for a number of systems, particularly in the presence of Lewis acids and more recently in the reaction between boryl triflates and aryloxides.17 The presence of a calix[4]arene rather than a calix[6]arene is thought to be due to the presence of a small amount of the n = 4 macrocycle in the batch of the precursor used. In the case of [TiF4] (3 equiv.), reaction with L1H6 afforded, following extraction into MeCN, the orange/red complex [(TiF)2(μ-F)L1H]2·6.5MeCN (3·6.5MeCN). The molecular structure (CCDC 2009076†) is shown in Fig. 2, with selected bond lengths and angles given in the caption. The molecule lies on a centre of symmetry and so half is unique. Two distorted octahedral titanium centres are bound to each of the two L1H macrocycles, the latter being linked by H-bonds. The central core can be described as two Ti2F2 diamonds, which bridge the calixarenes, whilst two fluoride ions bridge the two diamonds. The Ti–F bonds are somewhat longer than those found in the [O, NPy, N]-bearing Ti complexes recently reported by Solan et al.18 The MeCNs containing N(1) and N(2) reside in the calixarene cavity.
0.4569(10)); component 2 rotated by 8.1802° around [−0.01 0.98 0.18] (reciprocal) or [0.20 0.92 0.33] (direct). The core of the complex can be described as two, singly vertex–vacant cubes, connected by a face, in which the Ti octahedra share edges. Both Ti(2) and Ti(2A) possess O(CH2)4Cl groups. The main core of 2 was found to be similar to that of [Ti(NCMe)(μ3-O)L(O)4TiCl(O(CH2)4Cl)]2–2[TiCl(NCMe)(L(O)3(On-Pr))]·11MeCN, a compound we have recently reported.14 The formation of 2 is thought to involve the ring opening of the THF, which has been reported in the literature for a number of systems, particularly in the presence of Lewis acids and more recently in the reaction between boryl triflates and aryloxides.17 The presence of a calix[4]arene rather than a calix[6]arene is thought to be due to the presence of a small amount of the n = 4 macrocycle in the batch of the precursor used. In the case of [TiF4] (3 equiv.), reaction with L1H6 afforded, following extraction into MeCN, the orange/red complex [(TiF)2(μ-F)L1H]2·6.5MeCN (3·6.5MeCN). The molecular structure (CCDC 2009076†) is shown in Fig. 2, with selected bond lengths and angles given in the caption. The molecule lies on a centre of symmetry and so half is unique. Two distorted octahedral titanium centres are bound to each of the two L1H macrocycles, the latter being linked by H-bonds. The central core can be described as two Ti2F2 diamonds, which bridge the calixarenes, whilst two fluoride ions bridge the two diamonds. The Ti–F bonds are somewhat longer than those found in the [O, NPy, N]-bearing Ti complexes recently reported by Solan et al.18 The MeCNs containing N(1) and N(2) reside in the calixarene cavity.
Use of p-tert-butylcalix[8]areneH8 L2H8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65) with a silicone grease derived complex [Ti(NCMe)Cl]2[Ti(μ-O)]2[OSi(CH3)2OSi(CH3)2O]L2] in which the grease replaces two chloride ligands.20
65) with a silicone grease derived complex [Ti(NCMe)Cl]2[Ti(μ-O)]2[OSi(CH3)2OSi(CH3)2O]L2] in which the grease replaces two chloride ligands.20
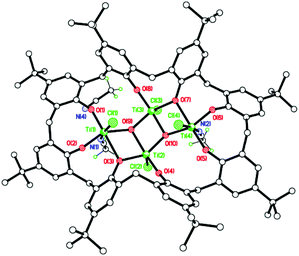
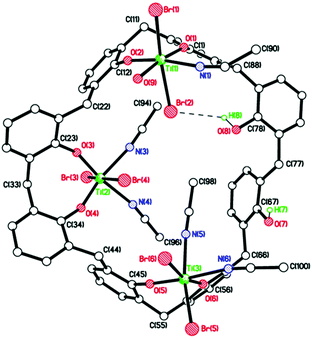
Some weak C–H⋯Cl intermolecular interactions parallel to a bind molecules into anti-parallel stacks (see ESI, Fig. S6†). In one preparation of 4, following work-up and crystallization from MeCN, the isolated crystals were identified as [Ti4O2Cl4(MeCN)2(L2)][Ti3Cl6(MeCN)5(OH2)(L2H2)][OH2]·11MeCN (5·11MeCN; CCDC 1973135†). The asymmetric unit of 5 contains 2 different molecules (Fig. 4). In one molecule there is a ladder structure, like 4, made up of four Ti(IV) ions and phenolate oxygens (from the L2 ligand) and bridging oxygens, O(3), O(7), O(9), and O(10). The latter two are oxo dianions. Ti(1) and Ti(4) each carry 1 Cl− ion and one MeCN ligand; Ti(2) and Ti(3) each carry one Cl− ion. In the other molecule, three Ti(IV) ions are coordinated to a L2H2 ligand via 6 phenolate oxygens (two per Ti); and oxygens O(17) and O(18) remain protonated. Ti(5) and Ti(6) are each coordinated to two Cl− ions and two MeCN ligands, and Ti(7) is coordinated to two Cl− ions, one MeCN ligand and one water molecule. There are two intramolecular H-bonds: O(18)–H(18)⋯O(17) and O(17)–H(17)⋯Cl(9), and a lone water molecule sits between the two titanium-calixarene molecules. The H atoms could not be located for this or the coordinated water molecule, but both appear to form reasonable H-bonds: O(19)⋯N(5) = 2.681, O(19)⋯O(20) = 2.759, and O(20)⋯Cl(1) = 3.525 Å. In terms of intermolecular interactions between molecules, the water molecule between the two different calix[8]arene complexes hydrogen bonds to the coordinated water molecule. The coordinated water molecule H-bonds to an acetonitrile molecule of crystallization.
Both coordinated MeCN groups point to the same side of the molecule, whilst the six MeCN molecules of crystallization are all exo to the complex. Molecules pack in layers. Adjacent molecules within layers adopt up–down–up–down orientations (see Fig. S7, ESI†). Between layers molecules stack in columns all pointing in the same direction with weak Br(1)⋯Br(2′) = 3.770 Å halogen bonding interactions. Given the addition of differing amounts of metal chloride to a calix[n]arene can be used to control the degree of metalation,8 we also investigated the addition of three equivalents of [TiBr4]. This resulted, following work-up (MeCN), in the formation of brown prisms for which a molecular structure determination revealed the asymmetric unit {[TiBr2(H2O)(NCMe)][TiBr2(NCMe)2Ti]2L2H2}·7.5(MeCN) 7·7.5(MeCN), see Fig. 6 (CCDC 1973131†). The calix[8]arene ligand retains two phenolic hydrogens on oxygens O(7) and O(8), which are not bound to titanium ions. Three TiBr2 moieties bind to the L2H2via two phenolate oxygens each. Each Ti ion has octahedral geometry. The bromides are trans on Ti(1) and Ti(2), but cis on Ti(3).
The remaining coordination sites are occupied by MeCN ligands in the case of Ti(2) and Ti(3), while Ti(1) bears one MeCN and most likely a water molecule. No peaks corresponding to carbon atoms were evident close to that water molecule that would have indicated MeCN. There is one intramolecular hydrogen bond between one of the phenol groups and one of the coordinated bromide ions. The other phenolic hydrogen does not make a hydrogen bond. Molecules are arranged in an undulating layer structure in the a/c plane (see Fig. S8,† ESI).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21
21
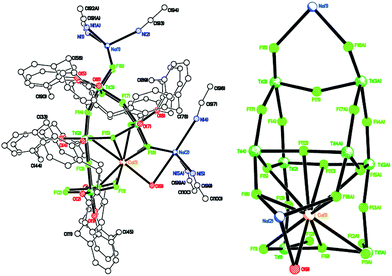 | ||
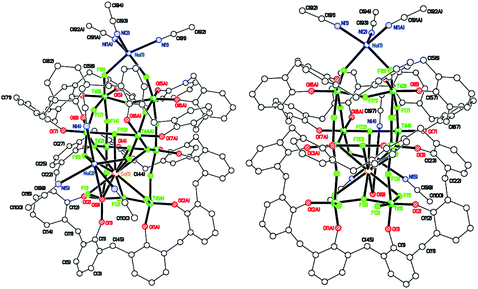
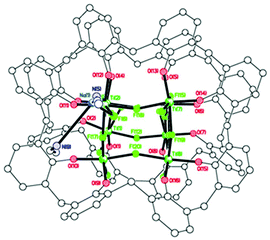
| Fig. 8 Side view (left) and core of the structure (right) of [Ti8CaF20(OH2)(Na2(MeCN)4)(L2)2]·14MeCN (8·14MeCN). | ||
We note that Ti–F complexes in which alkaline or alkaline-earth metal ions are featured in the structure in a host–guest fashion have been previously reported.22 However, these species were intentionally synthesized in a template-controlled manner, while the Na+ and Ca2+ ions present in 8 are likely to derive from the drying agents of the solvents used for the reaction/workup and are serendipitously incorporated into the structure.
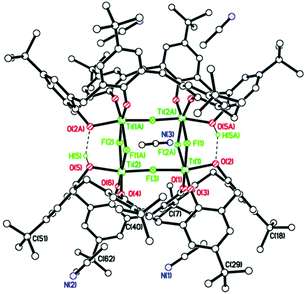
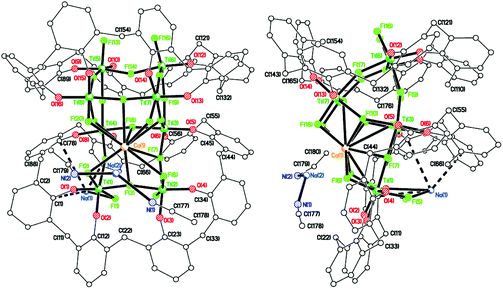
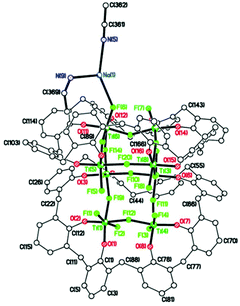
To investigate the reproducibility of such species, we repeated the reaction (using the same batch of L2H8) and again isolated red prisms. However, on this occasion the asymmetric unit (CCDC 1973365†) was found to be [Na(MeCN)2][Ti8CaF20NaO16(L2)2]·7MeCN (9·7MeCN), see Fig. 9, and unlike 8·14MeCN, it is not on a mirror plane, i.e. the whole molecule is unique, although there are also many similarities. The main core of the molecule again comprises 8 titanium ions and 20 fluoride ions with the fluorides a mixture of terminal and bridging Ti(3) and Ti(4) have 4 bridging fluorides, and Ti(5) > Ti(8) have 1 terminal and 3 bridging fluorides. Each octahedral titanium ion binds to an L2 ligand via two phenolate oxygens; each L2 binds to 4 titanium atoms. The oxygens bound to each individual titanium atom are cis. A calcium ion and two sodium ions are present to balance out the overall charge. The part of the core of the molecule containing the Ca2+ ion (see Fig. 10) is antifluorite-like with the Ca2+ coordinated by 11 F− ions, rather than the 9 in 8. Na(1) interacts with fluoride F(1), phenolate oxygen O(1), two ipso phenolate carbons C(1) and C(78), and the π-system of phenolate ring C(67) > C(72). Na(2) binds to two acetonitrile molecules, via N(1) and N(2), as a separate moiety in fairly close proximity to the Ca2+ ion and its coordinated fluorides.
Again, as in 8, four of the calixarene rings of each separate calixarene, bound to O atoms O(5) > O(8) on one, and O(9) > O(12) on the other, are close in space and adopt the same conformation, stacking almost exactly on top of each other. MeCN molecules and MeCN-solvated Na+ ions lie between titanocalix[8]arene complexes.
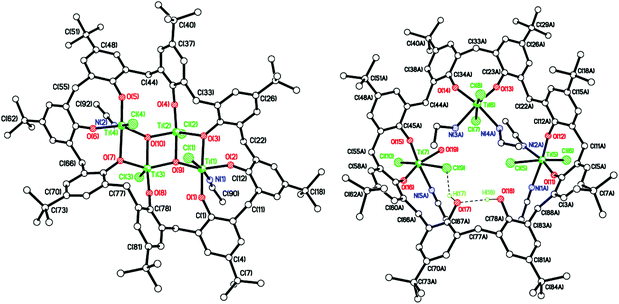
The complex [Na]6[Ti8F20Na(MeCN)2(L2)]-[Ti8F20Na(MeCN)0.5(L2)]·15.5(C2H3N) (10·15.5MeCN) has also been isolated and structurally characterized (see Fig. 11) from a re-run of this type of reaction, indicating that the products formed are variable and their exact nature is determined by the presence of drying agents in the solvents. For 10, there are negative charges: 2 × calix[8] = 16−, 20 × F− = 20−, total 36−; positive charges: 8 × Ti4+ = 32+, and 1 × Na+ gives a total of 33+. It is assumed that there are also another 3 Na+ ions to balance the charge and these are modelled by the Platon Squeeze procedure due to disorder and being randomly distributed between the MeCN molecules of crystallization.21 The amount of MeCN of crystallization should be regarded as approximate. There are two almost identical molecules in the asymmetric unit, differing only in the coordination site of the sodium ion and the number of acetonitrile molecules bonded to the sodium. The main core of each molecule is made up of 8 titanium ions and 20 fluorides as seen previously in 8 and 9 (see Fig. 12, and Fig. S11, ESI†). Two titanium ions have 2 terminal fluorides and 2 bridging fluorides, four titanium ions have 1 terminal fluoride and 3 bridging fluorides, and the remaining two titanium ions have 4 bridging fluorides. Each titanium ion binds to a L2 ligand via two phenolate oxygens; and each L2 binds to 4 titanium ions. Each Ti ion has octahedral geometry. The oxygens bound to each individual titanium atom are cis. In the first molecule, sodium ion Na(1) interacts with fluoride F(6) and two acetonitrile molecules including N(5) and N(9). In the second molecule, the major occupancy site of disordered sodium ion Na(2) interacts with fluorides F(25) and F(28), and the acetonitrile molecule including N(11), which was refined at half occupancy to match that of the major Na(2) component. Each pair of calixarene rings, on each titanium–fluoride core, are close in space and adopt the same conformation, stacking almost exactly on top of each other as seen previously (see Fig. 12 and S12, ESI†). MeCN molecules lie between titanocalixarene complexes. Also in this case, the Ti–F bonds for complexes 8–10 are slightly longer than those observed in previously reported compounds.18
Table 1 summarises the Ti–X bond length data from the four ladder structures described above. There are two main observations. Firstly, the Ti–X bond length increases by approx. 0.2 Å on going from Cl to Br and from Br to I, in line with the ionic radius increases as Group 17 is descended. Secondly, the Ti–X bond lengths for the halide attached to the six-coordinate end Ti ions, and trans to an MeCN nitrogen, is significantly longer (by 0.07–0.12 Å) than those for the apical halide attached to the central, five-coordinate, Ti ions with approx. square-based pyramidal geometry.
| Structure | X | Av. Ti–X for X on central Ti/Å | Av. Ti–X for X on end Ti/Å | Difference, Δ/Å |
|---|---|---|---|---|
| 4 & 5 | Cl | 2.173(2) | 2.297(2) | 0.124 |
| 6 | Br | 2.381(4) | 2.448(5) | 0.067 |
| 11 | I | 2.592(2) | 2.682(2) | 0.090 |
For catalysis comparison purposes, we have synthesised two diphenolate complexes bearing Br and I labile ligands, namely 12 and 13 (Scheme 1).
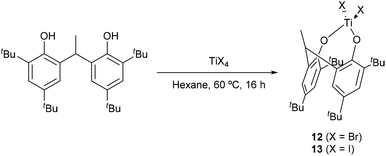 | ||
| Scheme 1 Synthesis of the diphenolate complexes 12 and 13.14 | ||
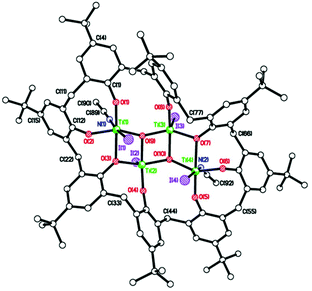
For the bromo-derivative 12, crystals suitable for X-ray analysis were obtained at room temperature from a saturated solution of the compound in hexane. The molecular structure of the complex (CCDC 2009076†) is shown in Fig. 14. The compound features a tetrahedral Ti4+ ion. The complex molecule and the hexane of crystallization both lie on a mirror plane, so half of the formula is unique. The dihedral angle between aromatic rings was found to be 64°. The hexane molecule lies in the cleft between the two aromatic rings. There is a weak intermolecular C–H⋯Br interaction between the methyl group at C(16) and Br(1) with an H(16A)⋯Br(1) distance of 3.05 Å.
ROP screening
| Run | Catalyst | ε-CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | Time (h) | Conv.a (%) | M n , | M n calc | M w/Mnb |
|---|---|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy on crude reaction mixture. b From GPC. c Values corrected considering Mark–Houwink factor (0.56) from polystyrene standards in THF. d Calculated from ([Monomer]0/[OH]0) × conv. (%) × Monomer molecular weight + Molecular weight of BnOH. e Reaction performed in air. | ||||||||
| 1 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
80 | 24 | None | — | — | — |
| 2 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 24 | 69 | 5240 | 6700 | 1.22 | |
| 3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 1 | 6.5 | — | — | — | |
| 4e | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 24 | 20 | 2690 | 3860 | 1.20 | |
| 5e | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 1 | None | — | — | — | |
| 6 | 3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
130 | 24 | None | — | — | — |
| 7 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | None | — | — | — |
| 8 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 8.4 | — | — | — | |
| 9 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | — | — | — | |
| 10 | 6 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | None | — | — | — |
| 11 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | None | — | — | — | |
| 12 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | — | — | — | |
| 13 | 9 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 64 | 8590 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230 |
1.63 |
| 14 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 15 | 11 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 74 | 6610 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
1.23 |
| 16 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 17 | 12 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | >99 | 6720 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
1.40 |
| 18e | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 5790 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
1.94 | |
| 19 | 13 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 42 | Liquid oligomers | ||
| 20 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 5640 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
1.30 | |
The bromo-(12) and iodo-(13) diphenolate titanium complexes were next investigated. Full conversion was achieved in the presence of the bromo derivative 12 at 80 °C within 24 h (run 17) affording a polymer with Mn of ca. 6.7 kDa and rather narrow polydispersity (1.40). Interestingly, complex 12 proved to be efficient also under aerobic conditions at 130 °C, but with less control (run 18). However, in both cases, the Mn were found to be much lower than the calculated values, suggesting the occurrence of transesterification processes. 1H NMR spectroscopic analysis on the sample isolated in run 16 highlighted the presence of signals at 7.33, 5.09 and 3.64 ppm in an integration ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, compatible with the presence of both BnO– and CH2OH end groups (Fig. S14, ESI†). This was further confimed by mass spectrometry. Indeed, the MALDI-ToF spectrum of the sample displayed a major series of peaks separated by 114 m/z units accountable to α-BnO-ω-OH terminated PCL n-mers as well as a minor population attributed to the corresponding Na+ adducts (Fig. S15, ESI†). The iodo-congener 13 was shown to be less efficient, affording 40% conversion at 80 °C (run 19) and low molecular weight oligomers. Interestingly, full conversion was achieved on increasing the temperature to 130 °C, affording a polymer of Mn 5.6 kDa with good control (Mw/Mn 1.30) (run 20). The higher activity of the diphenolate complexes can be ascribed to the increased accessibility of their metal centres compared with that of the calix[n]arene derivatives. However, 12 and 13 proved less active than related diphenolate species previously reported by Aida et al.24
2, compatible with the presence of both BnO– and CH2OH end groups (Fig. S14, ESI†). This was further confimed by mass spectrometry. Indeed, the MALDI-ToF spectrum of the sample displayed a major series of peaks separated by 114 m/z units accountable to α-BnO-ω-OH terminated PCL n-mers as well as a minor population attributed to the corresponding Na+ adducts (Fig. S15, ESI†). The iodo-congener 13 was shown to be less efficient, affording 40% conversion at 80 °C (run 19) and low molecular weight oligomers. Interestingly, full conversion was achieved on increasing the temperature to 130 °C, affording a polymer of Mn 5.6 kDa with good control (Mw/Mn 1.30) (run 20). The higher activity of the diphenolate complexes can be ascribed to the increased accessibility of their metal centres compared with that of the calix[n]arene derivatives. However, 12 and 13 proved less active than related diphenolate species previously reported by Aida et al.24
| Run | Catalyst | δ-VL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | Time (h) | Conversiona (%) | M n | M ncalc | M w/Mnb |
|---|---|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy on crude reaction mixture. b From GPC. c Calculated from ([Monomer]0/[OH]0) × conv. (%) × Monomer molecular weight + Molecular weight of BnOH. d Reaction performed in air. | ||||||||
| 1 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
80 | 24 | 18 | |||
| 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
80 | 1 | None | ||||
| 3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 24 | 45.8 | 6360 | 3930 | 1.13 | |
| 4d | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 24 | None | ||||
| 5 | 3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
130 | 24 | None | — | — | — |
| 6 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | None | |||
| 7 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | ||||
| 8 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | None | ||||
| 9 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 10 | 6 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 5.1 | |||
| 11 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | ||||
| 12 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 17 | ||||
| 13 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 14 | 9 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 65.4 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
1.43 |
| 15 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 16 | 11 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 69.6 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8820 | 1.37 |
| 17 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | None | ||||
| 18 | 12 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 52 | Oligomers | ||
| 19 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 64 | 6710 | 8120 | 1.92 | |
| 20 | 13 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 41 | Oligomers | ||
| 21 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 53 | 5590 | 6740 | 1.41 | |
In both cases, polymers with Mn lower than the calculated values were isolated; however, better control was exhibited by 13 compared with its bromo-congener (Mw/Mn 1.4 vs. 1.9).
| Run | Catalyst |
r-LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | Time (h) | Conversiona (%) | M n , | M ncalc | M w/Mnb |
|---|---|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy on crude reaction mixture. b From GPC. c Values corrected considering Mark–Houwink factor (0.58) from polystyrene standards in THF. d Calculated from ([Monomer]0/[OH]0) × conv. (%) × Monomer molecular weight + Molecular weight of BnOH. | ||||||||
| 1 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 24 | 87 | 8190 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.29 |
| 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 1 | 33 | ||||
| 3 | 3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
130 | 24 | None | — | — | — |
| 4 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | None | |||
| 5 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | None | ||||
| 6 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | None | ||||
| 7 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 8 | 6 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | None | |||
| 9 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | None | ||||
| 10 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | None | ||||
| 11 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 12 | 9 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | None | |||
| 13 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 14 | 11 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 8.2 | |||
| 15 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | ||||
| 16 | 12 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 4980 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.20 |
| 17 | 13 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 37 | Liquid oligomers | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VL ratio in the copolymer of 40
VL ratio in the copolymer of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60. No conversion was achieved when employing complex 3 (run 2). Low conversions, spanning from 20 to 40% were observed by using the titanocalix[8]arene complexes 4, 6, and 9 (runs 3–5). In all cases, the CL
60. No conversion was achieved when employing complex 3 (run 2). Low conversions, spanning from 20 to 40% were observed by using the titanocalix[8]arene complexes 4, 6, and 9 (runs 3–5). In all cases, the CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VL ratio was found to be ca. 1
VL ratio was found to be ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, the molecular weights of such co-polymers were too low to be detected by SEC, suggesting the occurrence of undesirable transesterification side-reactions resulting in the formation of light oligomers. Finally, good conversion was achieved by using 11 (81%, run 6). Similar to 1, the catalyst was shown to incorporate ε-CL and δ-VL in 1
1. However, the molecular weights of such co-polymers were too low to be detected by SEC, suggesting the occurrence of undesirable transesterification side-reactions resulting in the formation of light oligomers. Finally, good conversion was achieved by using 11 (81%, run 6). Similar to 1, the catalyst was shown to incorporate ε-CL and δ-VL in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The average sequence length for CL was found to be 2.46 while the value for VL was 1.69, as observed by 13C NMR spectroscopy (ESI, Fig. S17† and eqn (S1)–(S3)†).26 The randomness degree for the co-polymer was 1.0, compatible with a purely random copolymer.26a
1 ratio. The average sequence length for CL was found to be 2.46 while the value for VL was 1.69, as observed by 13C NMR spectroscopy (ESI, Fig. S17† and eqn (S1)–(S3)†).26 The randomness degree for the co-polymer was 1.0, compatible with a purely random copolymer.26a
| Run | Catalyst | ε-CL:δ-VL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
Conversiona (%) | CL/VLb | M n , | M w/Mnc |
|---|---|---|---|---|---|---|
| Reaction conditions: Toluene, T = 130 °C, 24 h.a Determined by 1H NMR spectroscopy on the crude reaction mixture based on ε-CL.b Determined by 13C NMR.c From GPC.d Values corrected considering Mark–Houwink factor [(Mn × %CL × 0.56) + (Mn × %VL)] from polystyrene standards in THF. | ||||||
| 1 | 1 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
67.6 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
7770 | 1.19 |
| 2 | 3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
None | — | — | — |
| 3 | 4 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29.9 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
nd | nd |
| 4 | 6 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
41.9 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
nd | nd |
| 5 | 9 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
22.2 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
nd | nd |
| 6 | 11 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81.0 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
5185 | 1.47 |
| 7 | 12 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
>99 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
1.70 |
| 8 | 13 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
>99 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
1.84 |
In the presence of the bi-phenolate complexes 12 and 13, complete conversion was observed (runs 7 and 8), affording co-polymers with Mn spanning from 12 to 14 kDa with rather poor control (Mw/Mnca.1.75). Also in this case, the co-polymer composition was analyzed by 13C NMR spectroscopy. For the polymer isolated with 12, the average sequence length was 2.10 and 2.05 for CL and VL, respectively (ESI, Fig. S18†), with a randomness degree of 0.97, compatible with a purely random co-polymer.26a A similar outcome was achieved with complex 13; in fact, the average sequence lengths were 2.50 and 1.82 for CL and CL, respectively, with a randomness degree of 0.95 (ESI, Fig. S19†).
Conclusion
The treatment of L1H6 with [TiCl4] afforded complex 1·4.5MeCN, in which two pseudo-octahedral titanium centres are bound to one calix[6]arene. A similar preparation conducted in THF resulted in the THF ring-opened product 2·4MeCN, containing an LH4- (where LH4 = p-tert-butylcalix[4]areneH4) derived ligand resulted from impurities in L1H6. In the case of [TiF4] (3 equiv.), reaction with L1H6 afforded the orange/red complex 3·6.5MeCN, in which two Ti2F2 diamonds bridge the calixarenes, whilst two fluoride ions bridge the two diamonds. The reaction between L2H8, with [TiCl4] led to [(TiCl)2(TiClNCMe)2(μ3-O)2(L2)]·1.5MeCN (4·1.5MeCN) and, from a similar preparation, to the co-crystallized complex [Ti4O2Cl4(MeCN)2(L2)][Ti3Cl6(MeCN)5(OH2)(L2H2)]·H2O·11MeCN (5·H2O 11MeCN). Extension of the L2H8 chemistry to [TiBr4] afforded, depending on the stoichiometry, [(TiBr)2(TiBrNCMe)2(μ3-O)2(L2)]·6MeCN (6·6MeCN) or [Ti(NCMe)2Br]2[Ti(O)Br2(NCMe)](L2)]·7.5MeCN (7·7.5MeCN), respectively. Interestingly, the use of [TiF4] afforded complexes containing Ca2+ and Na+, most likely deriving from drying agents, namely [Ti8CaF20(OH2)Na2(MeCN)4(L2)2]·14MeCN (8·14MeCN), [Na(MeCN)2][Ti8CaF20NaO16(L2)2]·7MeCN (9·7MeCN) or [Na]6[Ti8F20Na(MeCN)2(L2)][Ti8F20Na(MeCN)0.5(L2)]·15.5(C2H3N) (10·15.5MeCN). By using [TiI4], the ladder 11·7.25CH2Cl2 was isolated. These complexes have been tested as catalysts in the ring opening polymerization (ROP) of ε-CL, δ-VL and r-LA, both in air and N2. In the case of ε-CL, high temperatures (130 °C) over 24 h were required to achieve reasonable conversions for 1, 9 and 11 (3 and 6 were inactive). However, these metallocalix[n]arenes are out-performed by the diphenolates 12 and 13, which doubtless reflects the accessiblity of the metal centres in the latter. In the case of δ-VL, the salts 9 and 10 as well as 12 and 13 perform best, whilst for r-LA, 1, 12 and to a lesser extent 11, 13 were active. For the copolymerization of ε-CL with δ-VL reasonable activity was exhibited by 1 and 11, whilst conversions lower than 40% were observed with the other complexes; all afforded low molecular weight polymers. The copolymerization of ε-CL with r-LA was unsuccessful regardless of the catalyst employed. In general for these systems, more accessible metals centres (e.g.1, 12 and 13) and the formation of salts (e.g.9–11) favours improved catalytic performance in the ROP of cyclic esters.Experimental section
General
All manipulations were carried out under an atmosphere of dry nitrogen using conventional Schlenk and cannula techniques or in a conventional nitrogen-filled glove box. Hexane and toluene were refluxed over sodium. Acetonitrile was refluxed over calcium hydride. All solvents were distilled and degassed prior to use. IR spectra (nujol mulls, KBr windows) were recorded on a Nicolet Avatar 360 FT IR spectrometer; 1H NMR spectra were recorded at room temperature on a Varian VXR 400 S spectrometer at 400 MHz or a Gemini 300 NMR spectrometer or a Bruker Advance DPX-300 spectrometer at 300 MHz. The 1H NMR spectra were calibrated against the residual protio impurity of the deuterated solvent. Elemental analyses were performed by the elemental analysis service at the London Metropolitan University and in the Department of Chemistry, the University of Hull. All chemicals were purchased from either Sigma Aldrich or TCI UK.Synthesis of [Ti2Cl3(MeCN)2(OH2)(L1H)] [Ti2Cl3(MeCN)3(L1H)]·4.5 MeCN (1·4.5 MeCN)
To L2H6 (2.00 g, 2.05 mmol) in toluene (30 mL) was added [TiCl4] (0.71 mL, 6.50 mmol) and the system was refluxed for 12 h. On cooling, the volatiles were removed in-vacuo, and the residue was extracted into MeCN (30 mL). On standing at ambient temperature (ca. 10 °C) for 2 days, orange crystals of 1 formed. Yield 1.80 g, 64%. Sample dried under reduced pressure for 12 h (−4.5 MeCN). C142H175Cl6N5O13Ti4 requires C 66.51, H 6.88, N 2.73%. Found C 65.59, H 7.22, N 2.21%. IR: 3596w, 2727w, 2326w, 1635w, 1596w, 1364m, 1296m, 1259s, 1208s, 1112s, 1096s, 1022s, 928, 884s, 859m, 799s, 766m. 1H NMR (C6D6): 1H NMR (CDCl3, 298 K) δ: 7.15–6.76 (m, 12H, arylH), 6.36 (s, 1H, arylOH), 6.20 (s, 1H, Ti-OH), 4.74 (d, 2H, J = 12 Hz, endo-CH2), 4.19 (d, 1H, J = 12 Hz, endo-CH2), 4.33 (d, 1H, J = 12 Hz, endo-CH2), 4.11 (d, 1H, J = 12 Hz, endo-CH2), 3.57 (d, 4H, J = 12 Hz, exo-CH2), 2.98 (d, 1H, J = 12 Hz, exo-CH2), 2.01 (s, 12H, 4 uncoordinated MeCN), 1.29–1.01 (m, 54H, C(CH3)3), 0.50 (s, 6H, 2 coordinated MeCN).Synthesis of [Ti4Cl2(μ3-O)2(NCMe)2(L)2(O(CH2)4Cl)2]·4MeCN (2·4MeCN)
Crystals of complex 2 suitable for X ray analysis were obtained in low yield (<5%) from the preparation of 1. Due to the limited amount of sample (∼5 mg), only 1H NMR spectroscopy analysis could be performed. 1H NMR (C6D6) δ: 7.20–7.05 (m, 16H arylH), 5.43 (d, 4H, J = 13 Hz, endo-CH2), 5.15 (d, 4H, J = 13 Hz, endo-CH2), 4.97 (m, 4H, ClCH2CH2CH2CH2O–), 4.88 (m, 4H, ClCH2CH2CH2CH2O–), 4.10 (m, 4H exo-CH2), 2.24 (m, 4H, ClCH2CH2CH2CH2O–), 1.98 (m, 4H, ClCH2CH2CH2CH2O–), 1.56–1.23 (m, 72H C(CH3)).Synthesis of [(TiF)2(μ-F)L1H]2·6.5MeCN (3·6.5MeCN)
To L2H6 (2.00 g, 2.05 mmol) in toluene (30 mL) was added [TiF4] (0.76 g, 6.14 mmol) and the system was refluxed for 12 h. On cooling, the volatiles were removed in-vacuo, and the residue was extracted into MeCN (30 mL). On standing at ambient temperature at 0 °C for 2 days, orange/red crystals of 3 formed. Yield 1.75 g, 68%. C132H158F6O12Ti4·3MeCN (sample dried in vacuo for 2 h, −3.5MeCN) requires C 70.07, H 7.12, N 1.78%. Found C 68.82, H 7.18, N 1.29%. IR: 3442bs, 2727w, 2671w, 1636m, 1600m, 1417m, 1392s, 1364s, 1297m, 1260s, 1201s, 1101s, 1020s, 932m, 886m, 860m, 799s, 767w, 680w, 588w, 559w, 545w, 463w, 438w.1H NMR (CDCl3) δ: 7.24 (s, 8H, ArH), 7.12 (s, 8H, ArH), 6.99 (s, 8H, ArH), 5.17 (d, J = 12 Hz, 15H, endo-CH2), 4.00 (bs, 2H, –OH), 3.18 (d, J = 15 Hz, 12H, exo-CH2), 2.00 (s, 18H, 6 coordinated MeCN), 1.26 (s, 54H, C(CH3)), 1.16 (s, 54H, C(CH3)). 19F NMR (CDCl3) δ: −2.13 (q, J = 49 Hz, 2F, Ti–F–Ti), −15.96 (t, J = 46 Hz, 4F, TiF2).Synthesis of [(TiCl)2(TiClNCMe)2(μ3-O)2(L2)]·1.5MeCN (4·1.5MeCN)
As for 1, but using L2H8 (2.00 g, 1.54 mmol) and [TiCl4] (6.50 mL, 1.0 M in CH2Cl2, 6.50 mmol) affording 4 as small red prisms. Yield 2.13 g, 77%. Sample dried in value for 12 h (−1.5MeCN) C92H110Cl4N2O10Ti4 requires C 63.60, H 6.38, N 1.61%. Found C 62.87, H 6.71, N 1.43%. IR: 2319w, 2308w, 2289w, 2257w, 1648m, 1596s, 1393m, 1364m, 1291m, 1256s, 1196s, 1122m, 1105m, 1028m, 933s, 887s, 878s, 862s, 855s, 796m, 772w, 760w, 737w, 720m, 673w, 658w. 1H NMR (C6D6) δ: 7.29 (s, 4H, arylH), 7.08 (bs, 8H, arylH), 7.04 (bs, 4H, arylH), 5.62 (d, J = 12 Hz, 2H, endo-CH2), 5.11 (d, J = 12 Hz, 4H, endo-CH2), 4.21 (d, J = 12 Hz, 2H, endo-CH2), 3.87 (d, J = 12 Hz, 2H, exo-CH2), 3.40 (d, J = 12 Hz, 4H, exo-CH2), 2.68 (d, J = 12 Hz, 2H, exo-CH2), 1.12 (s, 36H, C(CH3)3), 1.04 (s, 36H, C(CH3)3), 0.50 (s, 27H, 9MeCN).Synthesis of [Ti4O2Cl4(MeCN)2(L2)][Ti3Cl6(MeCN)5(OH2)(L2H2)][OH2]·11MeCN (5·11MeCN)
As for 4, but using L2H8 (1.00 g, 0.77 mmol) and [TiCl4] (3.08 mL, 1.0 M in CH2Cl2, 3.08 mmol) affording 5 as small red prisms. Yield 1.03 g, 66%. Sample dried for 24 h in vacuo (−14MeCN) Ti7Cl10N7O19C190H233·OH2 requires C 63.07, H 6.50, N 1.60%. Found: C 62.87, H 6.71, N 1.43%. IR: 2313w, 2286w, 2249w, 1598w, 1577w, 1459m, 1416m, 1392s, 1364s, 1292s, 1259s, 1208s, 1158w, 1119m, 1102m, 1048w, 1024m, 964w, 948w, 929m, 886m, 874m, 860m, 832w, 816w, 794 m, 756w, 722 m, 699w. 1H NMR (C6D6) δ: 7.29 (s, 4H, arylH), 7.07 (bs, 8H, arylH), 7.03 (s, 4H, arylH), 5.62 (d, J = 13 Hz, 2H, endo-CH2), 5.08 (d, J = 13 Hz, 4H, endo-CH2), 4.17 (d, J = 13 Hz, 2H, endo-CH2), 3.87 (d, J = 13 Hz, 2H, exo-CH2), 3.38 (d, J = 13 Hz, 4H, exo-CH2), 2.66 (d, J = 13 Hz, 2H, exo-CH2), 1.22 (s, 36H, C(CH3)3), 1.03 (s, 36H, C(CH3)3), 0.50 (s, 3H, MeCN coordinated).Synthesis of [(TiBr)2(TiBrNCMe)2(μ3-O)2(L2)]·6MeCN (6·6MeCN)
As for 1, but using L2H8 (2.00 g, 1.54 mmol) and [TiBr4] (2.26 g, 6.16 mmol), affording 6 as orange/brown prisms. Yield 2.31 g, 69%. C92H110Br4N2O10Ti4·6MeCN requires C 57.79, H 5.97, N 5.19%. Found: C 58.77, H 6.39, N 4.88%. IR: 2361w, 2336w, 2313w, 2251w, 1633w, 1596w, 1415w, 1365s, 1291m, 1260s, 1198s, 1103s, 1026m, 932 m, 880s, 858s, 797s, 768w, 722 m, 684w. 1H NMR (C6D6; sample required heating for 3 h prior to running in order to increase solubility) δ: 7.07–7.02 (m, 16H, ArH), 5.70 (d, J = 13 Hz, 2H, endo-CH2), 5.18 (d, J = 13 Hz, 4H, endo-CH2), 4.22 (d, J = 13 Hz, 2H, endo-CH2), 3.86 (d, J = 13 Hz, 2H, exo-CH2), 3.42 (d, J = 13 Hz, 4H, exo-CH2), 2.64 (d, J = 13 Hz, 2H, exo-CH2), 2.05 (s, 1.5H, 0.5 uncoordinated MeCN), 1.21 (s, 36H, C(CH3)3), 1.10 (s, 36H, C(CH3)3), 0.54 (s, 18H, coordinated MeCN).Synthesis of [Ti(NCMe)2Br]2[Ti(OH2)Br2(NCMe)](L2)]·7.5MeCN (7·7.5MeCN)
As for 1, but using L2H8 (1.00 g, 0.77 mmol) and [TiBr4] (0.85 g, 2.31 mmol), affording 7 as orange/brown prisms. Yield 1.53 g, 81%. Sample dried under reduced pressure for 16 h (−6.5 MeCN). C98H123Br6N5O9Ti3·MeCN requires C 55.11, 5.83, N 3.86%. Found: C 56.89, H 5.74, N 3.78%. IR: 3380m, 2726w, 2671w, 2363w, 2342w, 2314w, 2286w, 2250w, 1744w, 1648w, 1597w, 1572w, 1377s, 1301w, 1288w, 1259m, 1201m, 1102m, 1028m, 934 m, 882 m, 872 m, 859 m, 799 m, 775w, 721w, 702w, 680w, 622w, 606w. 1H NMR (C6D6) δ: 7.17–6.96 (m, 16H, ArH), 5.75 (d, J = 12 Hz, 2H, endo-CH2), 5.23–5.39 (m, 2H, endo-CH2), 5.28 (d, J = 12 Hz, 2H, endo-CH2), 4.62 (d, J = 12 Hz, 2H, endo-CH2), 4.18 (m, 2H, exo-CH2), 4.01 (d, J = 12 Hz, 2H, exo-CH2), 3.64–3.50 (m, 2H exo-CH2), 3.39–3.34 (m, 2H, exo-CH2), 1.25 (s, 36H, C(CH3)3), 1.03 (s, 36H, C(CH3)3), 0.83 (s, 9H, coordinated MeCN). OH signals not found.Synthesis of [Ti8CaF20(OH2)(Na2(MeCN)4)(L2)2]·14MeCN (8·14MeCN)
L2H8 (2.00 g, 1.54 mmol) and [TiF4] (0.76 g, 6.16 mmol) were combined in toluene (30 mL) and the system was refluxed for 12 h. On cooling, volatiles were removed in-vacuo, and the residue was extracted into MeCN (30 mL). Prolonged standing at 0 °C afforded 8 as red, blade-like crystals. Yield: 1.87 g, 58%. The sample was dried under reduced pressure for 16 h. C184.67H234.02CaF20N4Na2O17Ti8·(−14MeCN) requires C 61.13, H 6.49, N 1.54%. Found: C 60.73, H 6.34, N 1.89%. IR: 1648w, 1303w, 1261s, 1199w, 1095s, 1020s, 929w, 873w, 859w, 800s, 753w, 722w, 660w. 1H NMR (C6D6) δ: 7.35–7.12 (m, 16H, ArH), 7.10–6.98 (m, 16H, ArH), 5.75 (m, 4H, endo-CH2), 5.50 (m, 8H, endo-CH2), 5.08–4.95 (m, 4H endo-CH2), 4.02–3.82 (m, 4H, exo-CH2), 3.43 (m, 4H, exo-CH2), 3.26 (m, 4H, exo-CH2), 3.08 (m, 4H, exo-CH2), 1.40–1.21 (m, 72H, C(CH3)3), 1.20–1.07 (m, 72H, C(CH3)3), 0.50 (s, 12H, 4 coordinated MeCN). 19F NMR (C6D6) δ: −4.25 (bs, 2F), −7.58 (bs), −8.89 (bs), −9.73 (bs) −11.1 (bs), −13.2 (bs), −17.2 (bs), −20.2 (bs), −22.1 (bs), −27.9 (bs).Synthesis of [Na(MeCN)2][Ti8CaF20NaO16(L2)2]·7(MeCN) (9·7MeCN)
As for 8, affording 9 as red blade-like crystals. Yield: 46%. C176H208CaF20Na2O16Ti8·9(C2H3N) requires C 61.41, H 6.21, N 2.64%. Found C 60.65, H 6.69, N 2.48%. IR: 1651w, 1300w, 1257s, 1200w, 1089s, 1015s, 925w, 871w, 857w, 798s, 756w, 727w, 663w. 1H NMR (C6D6) δ: 7.34–7.09 (m, 16H, ArH), 7.03–6.86 (m, 16H, ArH), 6.26–6.09 (m, 4H, endo-CH2), 5.94–5.80 (m, 8H, endo-CH2), 5.21–4.93 (m, 4H endo-CH2), 4.50–4.44 (m, 4H, exo-CH2), 4.40–4.35 (m, 4H, exo-CH2), 4.19–4.15(m, 4H, exo-CH2), 3.99–3.84 (m, 4H, exo-CH2), 2.11 (s, 21H, 7coordinated MeCN) 1.47–1.37 (m, 72H, C(CH3)3), 1.32–1.28 (m, 72H, C(CH3)3), 0.58 (s, 6H, 2 coordinated MeCN).19F NMR (C6D6) δ: −3.50 (bs, 2F), −6.72 (bs, 2F), −6.90 (bs, 2F), −8.04 (bs, 2F), −11.40 (bs, 2F), −13.74 (bs, 2F), −17.40 (bs, 2F), −19.61 (bs, 2F), −21.94 (bs, 2F), −27.32(bs, 2F).Synthesis of [Na]6[C180H214F20NaN2O16Ti8][C177H209.5F20NaN0.5O16Ti8]·15.5(C2H3N) (10·15.5MeCN)
As for 8, except that both the toluene and acetonitrile were dried over activated molecular sieves, to afforded 10 in 61% yield. Sample dried under reduced pressure for 3 h (−7.5MeCN). C357H423.5F40N2.5Na8O32Ti16·8(C2H3N) requires C 61.37, H 6.18, N 2.01%. Found: C 61.96, H 6.42, N 2.11%. IR: 2727w, 1598w, 1301m, 1260s, 1197m, 1020s, 929 m, 855 m, 798s, 752 m, 102w, 672w, 619 m, 590 m, 561 m, 541 m, 500 m. 1H NMR (CDCl3) δ: 7.64–7.57 (m, 16H, ArH), 7.30–7.26 (m, 16H, ArH), 4.67–4.62 (m, 4H, endo-CH2), 4.52–4.45 (m, 4H, endo-CH2), 3.99–3.96 (m, 8H endo-CH2), 3.44–3.99 (m, 8H, exo-CH2), 3.24–3.21 (m, 4H, exo-CH2), 2.94–2.90 (m, 4H, exo-CH2), 1.30–1.19 (m, 72H, C(CH3)3), 1.09–0.98 (m, 72H, C(CH3)3). 19F NMR (CDCl3δ: −3.78 (m, 2F), −6.78 (m, 1F), −8.61 (m, 2F), −10.0 (m, 2F), −11.7 (m, 4F), −13.5 (m, 2F), −15.2 (m, 1F), −19.9 (m, 2F), −23.2 (m, 2F), −32.3 (m, 2F).Synthesis of [(TiI)2(TiINCMe)2(μ3-O)2(L2)]·7.25CH2Cl2 (11·7.25CH2Cl2)
As for 1, but using L2H8 (2.00 g, 1.54 mmol) and [TiI4] (3.59 g, 6.46 mmol). Extraction into CH2Cl2 (30 mL) afforded upon prolonged standing at 0 °C red blocks of (11·7.25CH2Cl2). Yield: 2.22 g, 53%. C92H110I4N2O10Ti4·7.25(CH2Cl2) C 44.08, H 4.63, N 1.07%. Found: C 42.80, H 4.87, N 0.55%. IR: 2720w, 1650w, 1300, 1265m, 1190w, 1090m, 1015m. 920w, 870w, 859w, 790 m, 715w. 1H NMR (C6D6) δ: 7.31 (s, 4H, arylH), 7.10 (bs, 8H, arylH), 7.07 (bs, 4H, arylH), 5.70 (d, J = 12 Hz, 2H, endo-CH2), 5.08 (d, J = 12 Hz, 4H, endo-CH2), 4.15 (d, J = 12 Hz, 2H, endo-CH2), 3.84 (d, J = 12 Hz, 2H, exo-CH2), 3.48 (d, J = 12 Hz, 4H, exo-CH2), 2.63 (d, J = 12 Hz, 2H, exo-CH2), 1.08 (s, 36H, C(CH3)3), 1.00 (s, 36H, C(CH3)3).Synthesis of [Ti(Br)2(6,6′-(ethane-1,1-diyl)bis(2,4-di-tert-butylphenolate)]·hexane (12 hexane)
To a solution of 6,6′-(ethane-1,1-diyl)bis(2,4-di-tert-butylphenol) (1.00 g, 2.28 mmol) in hexane (30 mL), [TiBr4] (0.84 g, 2.28 mmol) was added and the mixture was stirred at 60 °C for 16 h. Upon cooling to room temperature, 12 was obtained as a red solid, which was recovered by filtration, washed with hexane (2 × 10 mL) and dried in vacuum at room temperature for 16 h (hexane). Yield 1.30 g, 88%. C30H44Br2O2Ti requires C 55.92, H 6.88%. Found C 55.69, H 7.05% 1H NMR (CDCl3) δ: 7.45 (d, J = 2.5 Hz, 2H, ArH), 7.20 (d, J = 2.5 Hz, 2H, ArH), 4.15 (q, J = 6.6 Hz, 1H, CH(CH3)), 1.74 (d, J = 6.9 Hz, 3H, CH3), 1.51 (s, 18H, C(CH3)3), 1.32 (s, 18H, C(CH3)3). IR: 1223m, 1198m, 1100 w, 925 m, 630 w, 480 m, 440 w, 415 m, 360 w. Single crystals suitable for X-ray diffraction were obtained from the mother liquor upon standing at room temperature for 2 days.Synthesis [Ti(I)2(6,6′-(ethane-1,1-diyl)bis(2,4-di-tert-butylphenolate)]·(13)
As for 11, but using 6,6′-(ethane-1,1-diyl)bis(2,4-di-tert-butylphenol) (0.50 g, 1.14 mmol) with [TiI4] (0.63 g, 1.14 mmol) in hexane (15 mL) affording 13 as a red solid. Yield 0.70 g, 83%. C30H44I2O2Ti requires C 48.80, H 6.01. Found C 49.43, H 6.66%. 1H NMR (CDCl3) δ: 7.45 (d, J = 2.3 Hz, 2H, ArH), 7.21 (d, J = 2.4 Hz, 2H, ArH), 3.92 (q, J = 6.6 Hz, 1H, CH(CH3)), 1.68 (d, J = 6.9 Hz, 3H, CH3), 1.58 (s, 18H, C(CH3)3), 1.32 (s, 18H, C(CH3)3). IR: 1225m, 1195m, 1090 w, 922 m, 625 w, 477 m, 443 w, 410 m, 363 w.Ring open polymerization (ROP) procedures
Typical polymerization procedures are as follows. Under nitrogen atmosphere, a Schlenk tube was charged with a toluene solution of the catalyst (10 mM) and the required amount of a toluene solution of benzyl alcohol (18 mM). The mixture was stirred for 2 min at room temperature, then the monomer (4.5 mmol) along with 1.5 mL toluene was added. The mixture was then placed into an oil bath pre-heated to the required temperature, and the solution was stirred for the prescribed time. The reaction was then quenched by addition of an excess of glacial acetic acid (0.2 mL) into the solution, and the resultant solution was then poured into methanol (200 mL). The resultant polymer was then collected on filter paper and dried in vacuo.Crystal structure determinations
Crystallographic data for all structures is summarized in Table 6 and the ESI,† and full details are provided in the deposited cifs. Diffraction data for all structures was collected using Cu-Kα radiation except 8·14MeCN and 12·C6H14 for which Mo-Kα radiation was used. A rotating anode X-ray source and Hypix 6000 detector Rigaku AFC11 diffractometer were employed in all cases.27 Data were corrected for Lp effects and for absorption.28 The structures were solved by a dual-space, charge flipping algorithm and refined by full matrix least-squares on F2 values.29,30 In common with most large tBuCalix[n]arene metal structures, disorder was observed in several tBu groups and the solvent of crystallisation. In each case the disorder was modelled with restraints in geometrical and anisotropic displacement parameters. When point atom modelling was no longer possible due to severe disorder problems, the Platon Squeeze procedure was used to model the affected regions as diffuse areas of electron density.21a,b Details of the specific disorder modelling employed for each structure is given in the ESI.† CCDC 1973130–1973136, 1973364–1973366, and 2009076–2009077 contain the supplementary crystallographic data for this paper.†| Compound | 1·4.5(C2H3N) | 2·4(C2H3N) | 3·6.5(C2H3N) | 4·1.5(C2H3N) |
|---|---|---|---|---|
| Formula | [C72H87Cl3N3O6Ti2] [C70H88Cl3N2O7Ti2]·4.5(C2H3N) | C100H126Cl4N2O12Ti4·4(C2H3N) | C132H158F6O12Ti4·6.5(C2H3N) | C92H110Cl4N2O10Ti4·1.5(C2H3N) |
| Formula weight | 2748.90 | 2045.64 | 2509.02 | 1798.79 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Orthorhombic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c | P212121 |
| a (Å) | 12.7071(4) | 11.1401(2) | 24.7656(7) | 18.7623(3) |
| b (Å) | 24.5925(4) | 15.00590(15) | 22.6045(5) | 19.3331(3) |
| c (Å) | 25.2999(7) | 16.5752(3) | 24.6564(6) | 26.9512(3) |
| α (°) | 104.399(2) | 104.2611(12) | 90 | 90 |
| β (°) | 96.177(2) | 93.0977(15) | 90.923(2) | 90 |
| γ (°) | 91.761(2) | 98.7122(13) | 90 | 90 |
| V (Å3) | 7599.8(3) | 2642.36(7) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 801.2(6) 801.2(6) |
9776.1(2) |
| Z | 2 | 1 | 4 | 4 |
| Temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) |
| Wavelength, λ (Å) | 1.54178 | 1.54178 | 0.71073 | 1.54178 |
| Calculated density (g cm−3) | 1.201 | 1.286 | 1.208 | 1.222 |
| Absorption coefficient, μ (mm−1) | 3.17 | 3.90 | 0.29 | 4.13 |
| Transmission factors (min./max.) | 0.694, 1.000 | 0.917, 1.000 | 0.568, 1.000 | 0.724, 1.000 |
| Crystal size (mm3) | 0.26 × 0.05 × 0.01 | 0.07 × 0.06 × 0.05 | 0.19 × 0.10 × 0.06 | 0.10 × 0.05 × 0.02 |
| θ(max) (°) | 68.2 | 68.3 | 27.5 | 68.2 |
| Reflections measured | 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465 465 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 296 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 785 785 |
| Unique reflections | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528 528 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 474 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792 792 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 801 801 |
| R int | 0.103 | 0.024 | 0.082 | 0.049 |
| Reflections with F2 > 2σ(F2) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 743 |
9515 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039 039 |
| Number of parameters | 1964 | 645 | 824 | 1175 |
| R 1 [F2 > 2σ(F2)] | 0.126 | 0.067 | 0.083 | 0.063 |
| wR2 (all data) | 0.380 | 0.190 | 0.245 | 0.180 |
| GOOF, S | 1.03 | 1.05 | 1.07 | 1.02 |
| Largest difference peak and hole (e Å−3) | 1.41 and −0.84 | 2.55 and −1.20 | 0.91 and −0.70 | 0.94 and −0.48 |
| Compound | 5·(H2O)·11(C2H3N) | 6·6(C2H3N) | 7·7.5(C2H3N) | 8·14(C2H3N) |
|---|---|---|---|---|
| Formula | [C92H110Cl4N2O10Ti4][C98H123Cl6N5O9Ti3]·OH2·11(C2H3N) | C92H110Br4N2O10Ti4·6(C2H3N) | C98H123Br6N5O9Ti3·7.5(C2H3N) | C184.67H234.02CaF20N4Na2O17Ti8·14(C2H3N) |
| Formula weight | 4078.23 | 2161.38 | 2446.07 | 4195.75 |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c | P21/n | P21/m |
| a (Å) | 18.0637(2) | 21.02305(10) | 21.5584(2) | 20.9093(17) |
| b (Å) | 20.6914(3) | 17.27686(8) | 26.9199(3) | 23.0191(7) |
| c (Å) | 31.5620(2) | 29.70228(14) | 21.6443(2) | 23.6904(10) |
| α (°) | 103.4502(9) | 90 | 90 | 90 |
| β (°) | 97.2290(8) | 102.5511(5) | 95.7763(7) | 99.755(5) |
| γ (°) | 99.0055(11) | 90 | 90 | 90 |
| V (Å3) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 168.9(2) 168.9(2) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530.42(9) 530.42(9) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497.5(2) 497.5(2) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237.6(11) 237.6(11) |
| Z | 2 | 4 | 4 | 2 |
| Temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) |
| Wavelength, λ (Å) | 1.54178 | 1.54178 | 1.54178 | 0.71073 |
| Calculated density (g cm−3) | 1.213 | 1.363 | 1.300 | 1.240 |
| Absorption coefficient, μ (mm−1) | 3.62 | 4.74 | 4.28 | 0.37 |
| Transmission factors (min./max.) | 0.757, 1.000 | 0.595, 1.000 | 0.539, 1.000 | 0.373, 1.000 |
| Crystal size (mm3) | 0.10 × 0.10 × 0.03 | 0.24 × 0.14 × 0.08 | 0.19 × 0.09 × 0.01 | 0.17 × 0.04 × 0.02 |
| θ(max) (°) | 68.3 | 68.3 | 68.2 | 27.5 |
| Reflections measured | 204![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131 131 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 958 958 |
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455 455 |
108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 461 461 |
| Unique reflections | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 624 624 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 224 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 797 797 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 376 376 |
| R int | 0.075 | 0.027 | 0.054 | 0.151 |
| Reflections with F2 > 2σ(F2) | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 722 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 352 352 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
8680 |
| Number of parameters | 2353 | 1278 | 1293 | 1389 |
| R 1 [F2 > 2σ(F2)] | 0.078 | 0.036 | 0.091 | 0.115 |
| wR2 (all data) | 0.242 | 0.095 | 0.293 | 0.399 |
| GOOF, S | 1.06 | 1.04 | 1.04 | 0.96 |
| Largest difference peak and hole (e Å−3) | 1.96 and −0.74 | 0.89 and −0.66 | 2.76 and −1.42 | 1.15 and −0.68 |
| Compound | 9·9(C2H3N) | 10·15.5(C2H3N) | 11·7.25CH2Cl2 | 12·hexane |
|---|---|---|---|---|
| Formula | C176H208CaF20Na2O16Ti8·9(C2H3N) | [Na]6[C180H214F20N2NaO16Ti8][C177H209.5F20N0.5NaO16Ti8]·15.5(C2H3N) | C92H110I4N2O10Ti4·7.25(CH2Cl2) | C30H44Br2O2Ti·C6H14 |
| Formula weight | 3798.16 | 7608.11 | 2718.73 | 730.54 |
| Crystal system | Monoclinic | Triclinic | Monoclinic | Orthorhombic |
| Space group | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c | Cmc21 |
| a (Å) | 23.4034(4) | 20.3323(2) | 29.2721(4) | 16.4524(3) |
| b (Å) | 22.4883(3) | 28.7880(3) | 9.52188(14) | 12.3781(2) |
| c (Å) | 37.6555(7) | 36.5201(3) | 43.6988(6) | 18.0939(3) |
| α (°) | 90 | 96.9496(8) | 90 | 90 |
| β (°) | 100.267(2) | 99.4528(9) | 103.0731(13) | 90 |
| γ (°) | 90 | 97.6499(9) | 90 | 90 |
| V (Å3) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500.9(6) 500.9(6) |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 673.9(4) 673.9(4) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 864.3(3) 864.3(3) |
3684.81(11) |
| Z | 4 | 2 | 4 | 4 |
| Temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) |
| Wavelength, λ (Å) | 1.54178 | 1.54178 | 1.54178 | 0.71073 |
| Calculated density (g cm−3) | 1.294 | 1.222 | 1.522 | 1.317 |
| Absorption coefficient, μ (mm−1) | 3.59 | 3.21 | 13.80 | 2.43 |
| Transmission factors (min./max.) | 0.662, 1.000 | 0.649, 1.000 | 0.241, 0.708 | 0.533, 1.000 |
| Crystal size (mm3) | 0.20 × 0.04 × 0.02 | 0.32 × 0.15 × 0.02 | 0.17 × 0.04 × 0.03 | 0.22 × 0.06 × 0.04 |
| θ(max) (°) | 68.2 | 68.3 | 68.3 | 28.7 |
| Reflections measured | 176![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468 468 |
340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 686 686 |
103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 662 662 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 640 |
| Unique reflections | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 581 581 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256 256 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509 509 |
4905 |
| R int | 0.100 | 0.096 | 0.152 | 0.037 |
| Reflections with F2 > 2σ (F2) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 907 907 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 212 212 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 795 795 |
4716 |
| Number of parameters | 2275 | 4870 | 1290 | 253 |
| R 1 [F2 > 2σ (F2)] | 0.084 | 0.099 | 0.099 | 0.027 |
| wR2 (all data) | 0.266 | 0.320 | 0.275 | 0.069 |
| GOOF, S | 1.02 | 1.03 | 1.04 | 1.05 |
| Largest difference peak and hole (e Å−3) | 0.92 and −0.71 | 1.32 and −0.55 | 1.56 and −1.89 | 0.54 and −0.72 |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
CR thanks the EPSRC for funding (EP/R023816/1), and the EPSRC National Crystallography Service at Southampton (UK) and the EPSRC Mass Spectrometry Service, Swansea (UK) for data.Notes and references
- See for example; (a) M. Delferro and T. J. Marks, Chem. Rev., 2011, 111, 2450 CrossRef CAS PubMed; (b) C. Redshaw, Catalysts, 2017, 7, 165 CrossRef.
- (a) D. M. Homden and C. Redshaw, Chem. Rev., 2008, 108, 5086 CrossRef CAS PubMed; (b) D. J. Hernández and I. Castillo, Current Trends in X-ray Crystallography, ed. A. Chandrasekaran, InTech, 2011. ISBN: 978-953-307-754-3 Search PubMed; (c) Y. Li, K.-Q. Zhao, C. Redshaw, B. A. Martínez Ortega, A. Y. Nuñez and T. A. Hanna, Coordination Chemistry and Applications of Phenolic Calixarene–metal Complexes, Patai's Chemistry of Functional Groups, Wiley, 2014.
- (a) S. Steyer, C. Jeunesse, D. Armspach, D. Matt and J. Harrowfield, in Calixarenes, ed. Z. Asfari, V. BÖhmer, J. Harrowfield and J. Vicens, Kluwer Academic Publishers, Dordrecht, 2001, ch. 28 Search PubMed; (b) C. Floriani and R. Floriani-Moro, Adv. Organomet. Chem., 2001, 47, 167 CrossRef CAS; (c) W. Sliwa, Croat. Chem. Acta, 2002, 75, 131 CAS; (d) P. D. Harvey, Coord. Chem. Rev., 2002, 233, 289 CrossRef; (e) A. J. Petrella and C. L. Raston, J. Organomet. Chem., 2004, 689, 4125 CrossRef CAS; (f) N. Kotzan and A. Vigalok, Supramol. Chem., 2008, 20, 129 CrossRef; (g) S. Siddiqui and P. J. Cragg, Mini-Rev. Org. Chem., 2009, 4, 283 CrossRef.
- (a) C. D. Gutsche, Calixarenes, The Royal Society of Chemistry, Cambridge, England, 1989 Search PubMed; (b) A. Arduini and A. Casnati, in Macrocycle Synthesis, ed. D. Parker, Oxford University Press, 1996, ch. 7 Search PubMed.
- C. Redshaw, Coord. Chem. Rev., 2003, 244, 45 CrossRef CAS.
- For early examples see. (a) M. M. Olmstead, G. Sigel, H. Hope, X. Xu and P. P. Power, J. Am. Chem. Soc., 1985, 107, 8087 CrossRef CAS; (b) A. Zanotti-Gerosa, E. Solari, L. Giannini, C. Floriani, N. Re, A. Chiesi-Villa and C. Rizzoli, Inorg. Chim. Acta, 1998, 270, 298 CrossRef CAS; (c) W. Clegg, M. R. J. Elsegood, V. C. Gibson and C. Redshaw, Dalton Trans., 1998, 3037 RSC; (d) U. Radius, Inorg. Chem., 2001, 40, 6637 CrossRef CAS PubMed; (e) F. A. Cotton, E. V. Dikarev, C. A. Murillo and M. A. Petrukhina, Inorg. Chim. Acta, 2002, 332, 41 CrossRef.
- (a) C. Redshaw and M. R. J. Elsegood, Inorg. Chem., 2000, 39, 5164–5168 CrossRef CAS PubMed; (b) C. Redshaw and M. R. J. Elsegood, Polyhedron, 2000, 19, 2657 CrossRef CAS; (c) V. C. Gibson, C. Redshaw and M. R. J. Elsegood, J. Chem. Soc., Dalton Trans., 2001, 767 RSC; (d) C. Redshaw and M. R. J. Elsegood, Eur. J. Inorg. Chem., 2003, 2071 CrossRef CAS; (e) C. Redshaw, D. H. Homden, D. L. Hughes, J. A. Wright and M. R. J. Elsegood, Dalton Trans., 2009, 1231 RSC; (f) C. Redshaw, M. Walton, K. Michiue, Y. Chao, A. Walton, P. Elo, V. Sumerin, C. Jiang and M. R. J. Elsegood, Dalton Trans., 2015, 44, 12292 RSC.
- V. C. Gibson, C. Redshaw and M. R. J. Elsegood, Chem. Commun., 2002, 1200 RSC.
- C. Redshaw, M. J. Walton, D. S. Lee, C. Jiang, M. R. J. Elsegood and K. Michiue, Chem. – Eur. J., 2015, 21, 5199 CrossRef CAS PubMed.
- Y. Li, K.-Q. Zhao, C. Feng, M. R. J. Elsegood, T. J. Prior, X. Sun and C. Redshaw, Dalton Trans., 2014, 43, 13612 RSC.
- J. D. Ryan, K. J. Gagnon, S. J. Teat and R. D. McIntosh, Chem. Commun., 2016, 52, 9071 RSC.
- M. Frediani, D. Sémeril, D. Matt, L. Rosi, P. Frediani, F. Rizzolo and A. M. Papini, Int. J. Polym. Sci., 2010, 490724 Search PubMed , 6 pages.
- M. Frediani, D. Sémeril, A. Marrioti, L. Rosi, P. Frediani, L. Rosi, D. Matt and L. Toupet, Macromol. Rapid Commun., 2008, 209, 1554 CrossRef.
- Z. Sun, Y. Zhao, O. Santoro, M. R. J. Elsegood, E. V. Bedwell, K. Zahra, A. Walton and C. Redshaw, Catal.: Sci. Technol., 2020, 10, 1619 RSC.
- O. Santoro and C. Redshaw, Catalysts, 2020, 10, 210 CrossRef CAS.
- C. Redshaw, M. Rowan, D. M. Homden, M. R. J. Elsegood, T. Yamato and C. Pèrez-Casas, Chem. – Eur. J., 2007, 13, 10129 CrossRef PubMed.
- (a) Y. Yu, Y. Zhang and R. Ling, Synth. Commun., 1993, 23, 1973 CrossRef CAS; (b) A. Solovyev, E. Lacôte and D. P. Curran, Dalton Trans., 2013, 42, 695 RSC; (c) S. L. Borkowsky, R. F. Jordan and G. D. Hinch, Organometallics, 1991, 10, 1268 CrossRef CAS; (d) T. L. Breen and D. W. Stephan, Inorg. Chem., 1992, 31, 4019 CrossRef CAS; (e) J. P. Campbell and W. L. Gladfelter, Inorg. Chem., 1997, 36, 4094 CrossRef CAS; (f) A. Mommertz, R. Leo, W. Massa, K. Harms and K. Dehnicke, Z. Anorg. Allg. Chem., 1998, 624, 1647 CrossRef CAS; (g) B. Birkmann, T. Voss, S. J. Geier, M. Ullrich, G. Kehr, G. Erker and D. W. Stephan, Organometallics, 2010, 29, 5310 CrossRef CAS.
- L. A. Wright, E. G. Hope, G. A. Solan, W. B. Cross and K. Singh, Organometallics, 2016, 35, 1183 CrossRef CAS.
- (a) G. D. Andreetti, G. Calestani, F. Ugozzoli, A. Arduini, E. Ghidini, A. Pochini and R. Ungaro, J. Inclusion Phenom., 1987, 5, 123 CrossRef CAS; (b) W. Clegg, M. R. J. Clegg, S. J. Teat, C. Redshaw and V. C. Gibson, J. Chem. Soc., Dalton Trans., 1998, 3037 RSC.
- D. M. Miller-Shakesby, S. Nigam, D. L. Hughes, E. Lopez-Estelles, M. R. J. Elsegood, C. J. Cawthorne, S. J. Archibald and C. Redshaw, Dalton Trans., 2018, 47, 8992 RSC.
- (a) P. v. d. Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194 CrossRef; (b) A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9 CrossRef CAS PubMed.
- (a) H. W. Roesky, M. Sotoodeh and M. Noltemeyer, Angew. Chem., Int. Ed. Engl., 1992, 31, 864 CrossRef; (b) A. Pevec, A. Demsar, V. Gramlich, S. Petricek and H. W. Roesky, J. Chem. Soc., Dalton Trans., 1997, 151, 2215 RSC; (c) F. Q. Liu, D. Stalke and H. W. Roesky, Angew. Chem., Int. Ed. Engl., 1995, 34, 1872 CrossRef CAS.
- A. Arbaoui, C. Redshaw and D. L. Hughes, Chem. Commun., 2008, 4717 RSC.
- D. Takeuchi, T. Nakamura and T. Aida, Macromolecules, 2000, 33, 725 CrossRef CAS.
- (a) C. Ludwig and M. R. Viant, Phytochem. Anal., 2010, 21, 22 CrossRef CAS PubMed; (b) M. J. Walton, S. J. Lancaster and C. Redshaw, ChemCatChem, 2014, 6, 1892 CrossRef CAS.
- (a) Q. Hu, S.-Y. Jie, P. Braunstein and B.-G. Lia, Chin. J. Polym. Sci., 2020, 38, 240 CrossRef CAS; (b) M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217 CrossRef CAS; (c) T. Wu, Z. Wei, Y. Ren, Y. Yu, X. Leng and Y. Li, Polym. Degrad. Stab., 2018, 155, 173 CrossRef CAS; (d) M. T. Hunley, N. Sari and K. L. Beers, ACS Macro Lett., 2013, 2, 375 CrossRef CAS.
- CrysAlis PRO, Rigaku Oxford Diffraction, 2017–2019 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- (a) P. v. d. Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194 CrossRef; (b) A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1973130–1973136, 1973364–1973366, 2009076, and 2009077. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt02130j |
| This journal is © The Royal Society of Chemistry 2020 |