Cu2O nanoparticles sensitize TiO2/CdS nanowire arrays to prolong charge carrier lifetime and highly enhance unassisted photoelectrochemical hydrogen generation with 4.3% efficiency†
Abstract
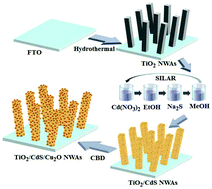
Effective separation of charge carriers and substantial prolongation of charge carrier lifetime are two vital issues for a photoanode to achieve highly efficient photoelectrochemical (PEC) hydrogen generation. Herein, a Cu2O-nanoparticle-sensitized TiO2/CdS (TiO2/CdS/Cu2O) nanowire array photoanode is fabricated via subtly combining successive ionic layer adsorption and reaction with a chemical bath deposition method. Both the type-II band alignment with a stair-like structure and a p–n junction are integrated into this ternary photoanode. The fabricated photoanode shows a significant enhancement in the PEC H2 production with 4.3% efficiency. The measurements of light absorption, photoluminescence and electrochemical spectra undoubtedly demonstrate that the enhanced PEC H2 generation is ascribed to the remarkable enhancement of visible-light absorbing ability, efficient space charge separation and substantial prolongation of charge carrier lifetime, which are achieved by the synergetic effects of the type-II band alignment with a stair-like structure and the p–n junction. The enhanced PEC H2 generation demonstrates the potential of the TiO2/CdS/Cu2O nanowire arrays as a photoanode to efficiently convert solar energy into chemical fuels.


 Please wait while we load your content...
Please wait while we load your content...