Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterisation of light lanthanide bis-phospholyl borohydride complexes†
Jingjing
Liu
 ,
Lydia E.
Nodaraki
,
Philip J.
Cobb
,
Lydia E.
Nodaraki
,
Philip J.
Cobb
 ,
Marcus J.
Giansiracusa
,
Marcus J.
Giansiracusa
 ,
Fabrizio
Ortu
,
Fabrizio
Ortu
 ,
Floriana
Tuna
,
Floriana
Tuna
 and
David P.
Mills
and
David P.
Mills
 *
*
Department of Chemistry, School of Natural Sciences, The University of Manchester, Manchester, M13 9PL, UK. E-mail: david.mills@manchester.ac.uk
First published on 28th April 2020
Abstract
Organometallic lanthanide (Ln) chemistry is dominated by complexes that contain substituted cyclopentadienyl (CpR) ligands. Closely related phospholyls have received less attention, and although they have proven utility in stabilising low oxidation state Ln complexes the trivalent Ln chemistry of these ligands is limited in comparison. Herein, we synthesise two families of heteroleptic Ln3+ complexes, [Ln(Htp)2(μ-BH4)]2 (Htp = 2,5-di-tert-butylphospholyl; 1-Ln; Ln = La, Ce, Nd, Sm), and [[Ln(Htp)2(μ-BH4)2K(S)]n (2-Ln, Ln = La, Ce, S = 2 DME, n = 2; 3-Ce, Ln = Ce, S = Et2O and THF, n = ∞) via the reactions of parent [Ln(BH4)3(THF)3.5] with K(Htp), to investigate differences between Ln complexes with substituted phospholyl ligands and analogous CpR complexes. Complexes 1–3-Ln were characterised as appropriate by single crystal XRD, SQUID magnetometry, elemental analysis, multinuclear NMR, ATR-IR and UV-Vis-NIR spectroscopy. Ab initio calculations reveal that small changes in the Ln3+ coordination spheres of these complexes can have relatively large influences on crystal field splitting.
Introduction
Although molecular lanthanide (Ln) chemistry lags behind that of the d-block, the remarkable physical properties and technological importance of Ln elements is now driving more rapid developments.1 Since the first examples of Ln cyclopentadienyl (Cp) complexes, [Ln(Cp)3], were isolated in 1954,2 Cp ligands and their derivatives (CpR) have dominated Ln organometallic chemistry.3,4 In the interim, phospholyl ligands have proven to be useful alternatives to CpR ligands in Ln chemistry and tend to exhibit η5-binding modes with these metals.5 Recent studies have shown that phospholyls are especially effective at stabilising divalent Ln complexes due to them being less electron-donating than their corresponding CpR ligands.6 The presence of a phosphorus lone pair can also promote the η1-binding mode and affect reactivity profiles, and 100% abundant 31P nuclei can provide a useful spectroscopic handle.5 However, despite these advantageous properties, trivalent Ln phospholyl chemistry is relatively underdeveloped.To date, there are only a handful of structurally authenticated examples of Ln3+ phospholyl complexes. In 1989, Nief and co-workers reported two examples of bis-phospholyl Ln3+ ‘ate’ complexes, [Ln(Tmp)2(μ-Cl)2(Li)(S)2] (Tmp = PC4Me4; Ln = Y, S = DME; Ln = Lu, S = Et2O) via the salt metathesis reactions of LnCl3 with two equivalents of Li(Tmp).7 The analogous bis-borohydride ‘ate’ complex, [Nd(Tmp)2(μ-BH4)2(K)(THF)], was later obtained by the use of [Nd(BH4)3(THF)3] and K(Tmp) as starting materials.8 The employment of LnI3 precursors yielded alkali metal salt-free monomeric [Ln(Dtp)2(I)] (Ln = Dy, Tm; Dtp = PC4tBu2-2,5-Me2-3,4) and dimeric [Tm(Htp)2(μ-I)]2 (Htp = PC4H2tBu2-2,5) or [Dy(Dsp)2(μ-I)]2 (Dsp = PC4(SiMe3)2-2,5-Me2-3,4), with the degree of aggregation dependent upon the steric bulk of the phospholyl ligand.9,10 In 1995, Nief attempted to prepare a trisubstituted Sm phospholyl complex by employing the less bulky Dmp (PC4H2Me2-2,5); dimeric [Sm(η5-Dmp)2(μ,η5:η1-Dmp)]2 was isolated.11 In 2016, Jaroschik et al. reported the first example of a monomeric tris-phospholyl Ln3+ complex, [Tm(η5-Dtp)2(η1-Dtp)], which was synthesised by the oxidation of [Tm(Dtp)2] with [Pb(Dtp)2].12 One Dtp ligand in this trivalent complex exhibits an η1-binding mode through the phosphorus lone pair; this is attributed to the steric demands of Dtp, and this motif contrasts with all η5-coordination for bulky CpR ligands on smaller Ln3+ ions, e.g. [Er(Cp*)3].13 Recently, our group showed that the bis-phospholyl Dy3+ complex, [Dy(Dtp)2][Al{OC(CF3)3}4], prepared by the sequential reaction of [Dy(Dtp)2(I)] with Mg(C3H5)Cl followed by [NEt3H][Al{OC(CF3)3}4], was shown to be a single-molecule magnet (SMM) exhibiting magnetic hysteresis up to 48 K.14
We targeted complexes of the general formula [Ln(Htp)3] for the largest Ln3+ cations, in an effort to prepare structurally similar analogues of [Ln(Cptt)3] (Ln = La, Ce, Nd, Sm; Cptt = C5H3tBu2-1,3),15 focusing on Kramers ions and diamagnetic La3+ analogues. However, we were unable to synthesise homoleptic complexes by the salt metathesis methods outlined herein and two families of heteroleptic complexes, [Ln(Htp)2(μ-BH4)]2 (1-Ln; Ln = La, Ce, Nd, Sm) and [Ln(Htp)2(μ-BH4)2K(S)2]n (2-Ln; Ln = La, Ce, S = DME, n = 2; 3-Ce, Ln = Ce, S = Et2O and THF, n = ∞), were characterised in this work by a variety of analytical techniques and ab initio calculations.
Results and discussion
Synthesis
The heteroleptic complexes 1-Ln (Ln = La, Ce, Nd, Sm) were prepared from the parent [Ln(BH4)3(THF)3.5]16 and two equivalents of K(Htp)17 by modification of the synthesis of [Tm(Htp)2(μ-I)]2, using TmI3 and K(Htp) (Scheme 1).9 Di-n-butyl ether was selected as the reaction solvent as its high boiling point (140.8 °C) allowed the reaction mixture to be heated significantly to increase the solubility of K(Htp). The crystalline yields for 1-La, 1-Ce, 1-Nd and 1-Sm were 31%, 41%, 15% and 7%, respectively, indicating that these salt metathesis reactions tend to become more sluggish for smaller Ln3+ cations. We were able to monitor the formation of diamagnetic 1-La by 31P NMR spectroscopy; the reaction appeared to proceed relatively cleanly but sluggishly, with only 1-La, K(htp) and a byproduct (Htp)2 present in appreciable quantities, thus we postulate that the low yields of 1-Ln were due to the loss of material during recrystallization processes. The especially low isolated yields of 1-Nd and 1-Sm were attributed to the ready formation of (Htp)2, which was observed in the 31P NMR spectra of all reaction mixtures but appeared to form in greater quantities for the smaller Ln. The formation of (Htp)2 in salt metathesis reactions has previously been seen in the synthesis of [Ga(Htp)] from the reaction of GaBr with one equivalent of Li(Htp).18The related complexes 2-Ln and 3-Ce were synthesised by analogous procedures using DME or diethyl ether, respectively, in the reactions of [Ln(BH4)3(THF)3.5] with two equivalents of K(Htp). The crystalline yields for 2-La, 2-Ce and 3-Ce were 16%, 46% and 31%, respectively; for 2-Ln the reaction mixtures were heated but in the case of 3-Ce the reaction was performed at room temperature. Although we are unable to conclusively determine if the differences between the degree of oligomerisation in 2-Ce and 3-Ce are due to the reaction temperature as the boiling point of diethyl ether (34.6 °C) is far lower than that of DME (85 °C), it is evident that changing the solvent to diethyl ether has allowed the salt metathesis reaction to proceed at a lower temperature, which is important to note for future synthetic attempts.
In common with observations for the synthesis of 1-Ln above, the yield of 2-La was lower than 2-Ce, indicating that reaction vectors and crystallisation processes are highly sensitive to Ln3+ cation size, and that (Htp)2 formation is likely an issue when reaction mixtures are heated for an extended period of time. We did not adapt these methods to attempt to prepare Nd and Sm analogues for 2-Ln. Variations of reaction stoichiometries to three equivalents of K(Htp) and changes in temperature and reaction times to those outlined above did not provide homoleptic complexes. Complexes 1-Ln, 2-Ln or 3-Ce were isolated in similar crystalline yields to those stated above upon the variation of any of these parameters, though the amount of (Htp)2 formed in the reaction mixtures of 1-Nd and 1-Sm appeared to increase when these were heated for prolonged periods at elevated temperatures and monitored by 31P NMR spectroscopy.
Elemental analysis results consistently gave low carbon values, likely due to carbide formation from incomplete combustion, but all other analytical data were consistent with their bulk purity (see below); for 3-Ce elemental analysis values are in agreement with 1H NMR data where complete desolvation occurred when the sample was exposed to vacuum for 1 hour (1 × 10−2 mbar). The ATR-IR spectra of most complexes clearly exhibit absorptions from 2500 to 2100 cm−1 (see ESI Fig. S47–S55†); these are attributed to B–H vibrations by comparison to those reported for [Ln(Cptt)2(μ-BH4)]2 (Ln = La, Ce, Sm).16b,19 As expected the ATR-IR spectra of 2-La and 2-Ce are nearly superimposable, but 1-Ln fall into two distinct pairs, with analogous spectra for 1-La and 1-Sm, and for 1-Ce and 1-Nd; this observation is curious given that the single crystal XRD data indicate that 1-Ln are all structurally analogous in the solid state (see below).
NMR spectroscopy
1H NMR spectra were recorded from −350 to +350 ppm for 1-Ln, 2-Ln and 3-Ce, though paramagnetic shifts were relatively small for Ce, Nd and Sm analogues (Table 1). Two signals were observed in all spectra in a ratio of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; these correspond to the tBu groups and the Htp ring protons, respectively. Due to restricted rotation of the Htp rings in 2-La, we observed two tBu group resonances at 298 K; VT 1H NMR spectra in C7D8 from 218–318 K showed that these two signals coalesced at 318 K, and exhibited greater separation at lower temperatures with an approximate rotational energy barrier of 31(7) kJ mol−1 (see ESI†). The BH4− anions were not seen in the 1H NMR spectra of 1-Ln, 2-Ln and 3-Ce, even for diamagnetic 1-La and 2-La, but were observed by 11B{1H} NMR spectroscopy [δB: −20.89 (1-La), −3.90 (1-Ce), −71.18 (1-Sm), −22.70 and −20.89 (2-La), 13.35 (2-Ce)]. For 1-La B–H coupling (1JBH = 84.1 Hz) was observed in the 11B NMR spectrum; a similar coupling constant was previously seen for [La(Cptt)2(μ-BH4)]2 (1JBH = 81 Hz).16b
4; these correspond to the tBu groups and the Htp ring protons, respectively. Due to restricted rotation of the Htp rings in 2-La, we observed two tBu group resonances at 298 K; VT 1H NMR spectra in C7D8 from 218–318 K showed that these two signals coalesced at 318 K, and exhibited greater separation at lower temperatures with an approximate rotational energy barrier of 31(7) kJ mol−1 (see ESI†). The BH4− anions were not seen in the 1H NMR spectra of 1-Ln, 2-Ln and 3-Ce, even for diamagnetic 1-La and 2-La, but were observed by 11B{1H} NMR spectroscopy [δB: −20.89 (1-La), −3.90 (1-Ce), −71.18 (1-Sm), −22.70 and −20.89 (2-La), 13.35 (2-Ce)]. For 1-La B–H coupling (1JBH = 84.1 Hz) was observed in the 11B NMR spectrum; a similar coupling constant was previously seen for [La(Cptt)2(μ-BH4)]2 (1JBH = 81 Hz).16b
| Complex | δ 1H/ppm {PC4H2C(CH3)3} | δ 1H/ppm {PC4H2C(CH3)3} |
|---|---|---|
| 1-La | 1.52, 72H | 7.35, 8H |
| 1-Ce | −3.70, 72H | −2.64, 4H; −4.63, 4H |
| 1-Nd | −5.09, 72H | −13.86, 8H |
| 1-Sm | −0.54, 72H | 16.40, 8H |
| 2-La | 1.50, 36H; 1.75, 36H | 7.2–7.4, 8H |
| 2-Ce | −1.84, 24H; −3.68, 48H | 11.35, 8H |
| 3-Ce | −3.45, 36H | 1.44, 4H |
The paramagnetism of 1-Ce, 1-Nd, 1-Sm, 2-Ce and 3-Ce precluded assignment of their 13C{1H} NMR spectra, however, for diamagnetic 1-La and 2-La these could be interpreted: for 1-La the expected two tBu group resonances were observed at 34.50 and 37.02 ppm and the two Htp ring carbon environments were located at 125.01 and 178.08 ppm. The signals for carbon atoms on the Htp ring and tBu groups are doublets from coupling with 31P (1JPC = 59.7 Hz; 2JPC = 15.7 Hz; 3JPC = 6.9 Hz); similar coupling constants were previously seen for [Pb(Dtp)2] (1JPC = 45.8 Hz; 2JPC = 16.2 and 3.4 Hz) and [Pb(Dsp)2] (1JPC = 66.0 Hz; 2JPC = 3.0 Hz; 3JPC = 6.0 Hz).12 The Htp ring was only observed by 31P{1H} NMR spectroscopy for diamagnetic 1-La (δP: 105.65 ppm) and 2-La (δP: 96.49 ppm), however, for paramagnetic 1-Ln, 2-Ce and 3-Ce only diamagnetic impurities and (Htp)2 were observed at ca. −62 ppm and −24 ppm, respectively.
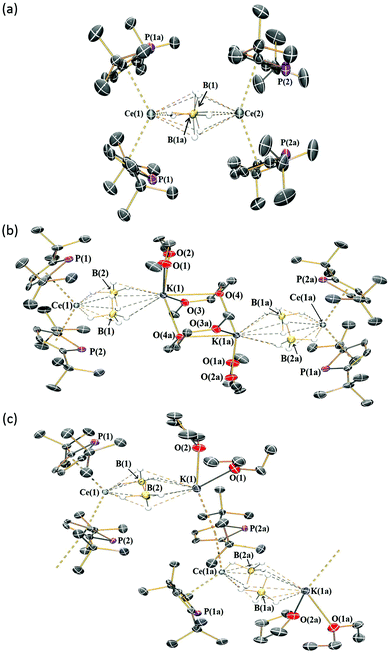
Crystallography
The solid-state structures of 1-Ln, 2-Ln and 3-Ce were determined by single crystal XRD (1-Ce, 2-Ce and 3-Ce are depicted in Fig. 1, selected bond distances and angles are compiled in Table 2; see ESI† for additional crystallographic data). Due to the poor data quality for 1-Nd metrical parameters are not included, however the data is of sufficient quality to provide connectivity. All Ln3+ cations in 1-Ln, 2-Ln and 3-Ce are capped with two η5-Htp ligands and have two equatorial BH4− anions. For the dinuclear 1-Ln series the BH4− anions bridge two Ln3+ cations, whereas for 2-Ln and 3-Ce these bridge one Ln3+ and one K+ cation.| Complex | Ln⋯Htpcent/Å | Htpcent⋯Ln⋯Htpcent/° | Ln⋯B/Å |
|---|---|---|---|
| 1-La (La(1)) | 2.634(7) | 123.7(2) | 2.855(13) |
| (La(2)) | 2.664(6) | 141.0(2) | 2.914(13) |
| 1-Ce (Ce(1)) | 2.605(6) | 123.5(2) | 2.904(12) |
| (Ce(2)) | 2.626(6) | 140.1(2) | 3.009(12) |
| 1-Sm (Sm(1)) | 2.544(6) | 124.7(2) | 2.806(16) |
| (Sm(2)) | 2.578(7) | 141.8(2) | 2.939(16) |
| 2-La | 2.6721(11), 2.6672(11) | 121.23(3) | 2.723(4), 2.724(3) |
| 2-Ce | 2.6288(18), 2.6337(18) | 121.63(6) | 2.698(5), 2.699(6) |
| 3-Ce | 2.632(2), 2.683(2) | 121.40(7) | 2.695(5), 2.705(7) |
In the case of 2-Ln the K+ cations are bound by two DME molecules, one of which bridges to the K+ cation of a second [Ln(Htp)2(μ-BH4)2K(DME)(μ-DME)] unit. In contrast, the K+ cations of 3-Ce are bound by one THF, one diethyl ether, and an η5-Htp that bridges to the Ce3+ centre of the next [Ce(Htp)2(μ-BH4)2K(THF)(Et2O)] unit, forming a coordination polymer. In all cases, we are not fully confident in assigning precise binding modes of BH4− anions from the combination of ATR-IR spectra and XRD data.
The mean Ln⋯B distances in 1-Ce (2.96(2) Å) and 1-Sm (2.87(2) Å) are comparable to those in [Ln(Cptt)2(μ-BH4)]2 (Ln = Ce, 2.93(2) Å; Ln = Sm, 2.858(8) Å),16b,19b which is unsurprising given the similarity in size of Htp and Cptt. The mean Ln⋯Htpcent distances decrease regularly across the 1-Ln series (e.g.1-La, 2.649(8) Å; 1-Sm, 2.561(9) Å); however, these are all larger than the corresponding Ln⋯Cpcent distances in [Ln(Cptt)2(μ-BH4)]2 (Ln = Ce, 2.535 Å; Ln = Sm, 2.46 Å).19
All the 1-Ln series exhibit two sets of Htpcent⋯Ln⋯Htpcent angles (Ln = La, 123.5(2)° and 141.2(2)°; Ln = Ce, 123.4(2)° and 140.2(2)°; Ln = Sm, 124.5(3)° and 141.8(3)°); these are all closer to linearity than the mean Cpttcent⋯Ln⋯Cpttcent angles in [Ln(Cptt)2(μ-BH4)]2 (Ln = Ce, 119.4°; Ln = Sm, 115.2°).19 The mean Ln⋯Htpcent distances in 2-La (2.670(2) Å) are slightly longer than those in 2-Ce (2.631(3) Å) but are statistically equivalent to those in 3-Ce (2.658(3) Å).
Electronic spectroscopy
The electronic spectra of 1-Ln, 2-Ln and 3-Ce were obtained at room temperature as ca. 0.5 mM solutions in toluene (UV-Vis-NIR spectra of 1-Ln compiled in Fig. 2; other spectra are shown in the ESI†). All complexes exhibited intense ligand–metal charge transfer bands, which tailed into the visible region from the UV to varying extents. Due to their Laporte-forbidden nature, f–f transitions are relatively weak;20 pale green 1-Nd exhibits an absorption at![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max = 16
max = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 cm−1 (ε = 390 mol−1 dm3 cm−1), owing to the 4I9/2 → 4G5/2 transitions.20 No f–f transitions could be observed for the other complexes. Complex 1-Ce showed two very broad but distinguishable absorptions at ca. 21
750 cm−1 (ε = 390 mol−1 dm3 cm−1), owing to the 4I9/2 → 4G5/2 transitions.20 No f–f transitions could be observed for the other complexes. Complex 1-Ce showed two very broad but distinguishable absorptions at ca. 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and 23
000 cm−1 and 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 with similar extinction coefficients (ε ≈ 200 mol−1 dm3 cm−1), whereas 2-Ce and 3-Ce showed similar broad absorptions at 21
000 cm−1 with similar extinction coefficients (ε ≈ 200 mol−1 dm3 cm−1), whereas 2-Ce and 3-Ce showed similar broad absorptions at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 cm−1 (ε = 200 mol−1 dm3 cm−1 and 250 mol−1 dm3 cm−1, respectively). These absorptions are assigned to [Xe]4f1 → [Xe]4f05d1 transitions, which are formally allowed by electric dipole selection rules, though the extinction coefficients are relatively small and are typically ca. 600–1200 mol−1 dm3 cm−1 for Ce3+ complexes; this is attributed to a combination of the low local symmetry and dipole–dipole effects.20
300 cm−1 (ε = 200 mol−1 dm3 cm−1 and 250 mol−1 dm3 cm−1, respectively). These absorptions are assigned to [Xe]4f1 → [Xe]4f05d1 transitions, which are formally allowed by electric dipole selection rules, though the extinction coefficients are relatively small and are typically ca. 600–1200 mol−1 dm3 cm−1 for Ce3+ complexes; this is attributed to a combination of the low local symmetry and dipole–dipole effects.20
 | ||
Fig. 2 UV-vis-NIR spectra of 1-Ln (ca. 0.5 mM in toluene) from 6000 to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 at room temperature. 000 cm−1 at room temperature. | ||
Magnetism
We were unable to collect reliable EPR spectroscopic data for paramagnetic 1-Ln, 2-Ln and 3-Ce as powders or as frozen toluene solutions. Multiple measurements were attempted at both X and K-band frequencies to probe the electronic structure and the presence of exchange interactions. The sample stability and weak signal, including issues with grinding polycrystalline samples, the presence of inequivalent Ln3+ sites and multiple hyperfine splittings (by 31P, 10B and 11B nuclei), proved problematic for obtaining reliable and reproducible spectra and as such discussion of these data is omitted. However, variable temperature DC magnetic measurements were recorded for all paramagnetic complexes as solids suspended in eicosane by SQUID magnetometry with 0.1 T applied magnetic field, or in solution at 298 K by the Evans method21 (see ESI† for full details). Relatively small discrepancies between solid and solution χMT values for all samples are attributed to weighing errors and the estimation of diamagnetic corrections. The magnetic susceptibility-temperature product (χMT) for solid 1-Ce at 300 K was 1.32 cm3 mol−1 K, which is similar to the solution value of 1.47 cm3 mol−1 K but lower than the expected value for a dinuclear Ce3+ complex of 1.60 cm3 mol−1 K (S = 1/2, L = 3, 2F5/2).1 For solid 1-Nd the 300 K χMT value of 2.68 cm3 mol−1 K is higher than the solution value of 2.02 cm3 mol−1 K but is lower than the calculated value of 3.26 cm3 mol−1 K for two non-interacting Nd3+ ions (S = 3/2, L = 6, 4I9/2). In contrast, the χMT product for 1-Sm at 300 K (0.61 cm3 mol−1 K) is consistent with the solution value (0.71 cm3 mol−1 K), but both are higher than the expected value of a dinuclear Sm3+ complex (0.18 cm3 mol−1 K; S = 5/2, L = 5, 6H5/2). Experimentally obtained χMT for Sm3+ complexes are consistently higher than free-ion values due to the mixing of low lying J multiplets.1The χMT values at 300 K for solid 2-Ce (1.16 cm3 mol−1 K) and 3-Ce (0.63 cm3 mol−1 K) are similar to solution moments (1.55 and 0.68 cm3 mol−1 K, respectively). A dinuclear formulation was used to calculate the moment of 2-Ce and a single Ce3+ centre was used in the calculation for polymeric 3-Ce; as a result the moment of 2-Ce is similar to 1-Ce, and that of 3-Ce is approximately half that of 1-Ce. Again these values are lower than the predicted free-ion χMT values (0.80 cm3 mol−1 K for a single Ce3+ ion; S = 1/2, L = 3, 2F5/2), consistent with the results obtained for 1-Ln. The low temperature magnetisation measurements of 1-Ce, 1-Nd and 1-Sm measured between 0–7 T at temperatures of 2 K and 4 K did not reach magnetic saturation, which can be ascribed to the large magnetic anisotropy of the system and/or to the presence of low-lying excited states.22 In contrast, the isothermal magnetisation curves for 2-Ce and 3-Ce at 2 K exhibit near-saturation at 7 T.
CASSCF-SO calculations
In order to probe the variation in the crystal field (CF) environments of 1-Ln, 2-Ln and 3-Ce, complete active-space self-consistent field spin–orbit (CASSCF-SO) calculations were performed with Molcas 8.023 using the X-ray crystal coordinates. For polymeric 3-Ce, a molecular fragment containing two K+ ions and a single Ce3+ ion was used, completing the ligand coordination sphere around each K+; see ESI† for full details. The CF splitting of the ground J Russell–Saunders terms are presented in Tables S5–S12.† The theoretically predicted magnetic properties were compared to the experimentally determined susceptibility and magnetisation curves. For 1-Ce, 1-Sm and 3-Ce, ab initio calculations are in good agreement with experimental data, whilst for all other paramagnetic complexes the experimentally obtained values are all lower than those calculated, as is also seen by Curie law comparison of the room temperature χMT. The strong CASSCF agreement for 1-Sm, which was not predicted by the Curie Law, arises from temperature independent paramagnetism owing to large CF splitting and a weakly magnetic ground doublet state.1For the 1-Ln series, the two inequivalent Ln3+ ion sites result in unique CF splitting of the ground free ion J multiplets (Tables S5–S10†). These sites can be distinguished by the orientation of the P atoms in the Htp rings with respect to the bridging BH4− moieties: Ln(1) refers to the smaller Htpcent⋯Ln⋯Htpcent angle where the P atoms are positioned relatively close to BH4−, whilst Ln(2) refers to the CF environment with the larger Htpcent⋯Ln⋯Htpcent angle where the P atoms are far away from BH4−. Consistently, Ln(2) has a larger overall CF splitting of the mJ sublevels, which follows the trend with linearity of the Htpcent⋯Ln⋯Htpcent angle. Complex 1-Ce reveals the most dramatic contrast between the two sites, with Ce(2) having ca. 50% larger CF splitting (892 cm−1) than Ce(1) (626 cm−1); this contrast is also observed for Nd and Sm but to a much smaller extent (<20% difference in CF splitting). As there is almost no variation in the Ln⋯Htpcent distances of the two sites, the CF splitting behaviour is likely a result of the coordination angle of the Htp ligands and influenced by the skewed electron density resulting from the position of the P atom within the Htp ring. There is ca. 0.1 Å difference between the Ln⋯B distances of each site from the bridging BH4− moieties across all variants. Here, the longer Ln⋯B interaction is always Ln(2), with the larger Htpcent⋯Ln⋯Htpcent angle.
For the Ce Htp complexes (Tables S5, S6, S11 and S12†), the coordination environment of Ce(1) from 1-Ce shows a similar bonding motif of the Htp ligands with 2-Ce and 3-Ce (Table 2). Despite the similar Ce⋯Htp bond lengths and angles, the BH4− anions are closer to the Ce3+ ions in 2-Ce and 3-Ce compared to 1-Ce (Ce⋯B ca. 2.7 vs. 2.9 Å), likely a result of the smaller neighbouring {K(S)2}+ coordination spheres instead of the sterically demanding {Ce(Htp)2}+ moiety. This closer interaction results in a significantly smaller CF splitting in 2-Ce and 3-Ce (285 and 320 cm−1, respectively) and provides a direct representation of the influence of the weak interactions from the BH4− anions. It has previously been shown that control and minimisation of equatorial anion interactions in isolated [Dy(CpR)2]+ and [Dy(Dtp)2]+ cations is of crucial importance in high-performance SMM design.14,24 Our results highlight that small variations to the equatorial ligand field in almost identical {Ln(Htp)2}+ moieties can have a relatively large influence on the CF splitting of Ln3+ ions, and hence a potentially significant impact on the resultant magnetic properties for late Ln3+ analogues.
As a further point of comparison, CASSCF-SO calculations were performed on the literature complex [Ce(Cptt)2(μ-BH4)]2 (4-Ce) (Tables S13 and S14†).25 This system has two unique Ce3+ coordination environments, albeit with minimal differences in metrical parameters (Cpttcent⋯Ln⋯Cpttcent angles of 119.2 and 119.6°, Ce⋯Cpttcent distances of 2.53 and 2.54 Å, and Ce⋯B distances of 2.93(2) Å). Interestingly, the coordination environment of 4-Ce is similar to Ce(1) from 1-Ce (with slightly shorter Ce⋯Cpttcent distances due to the less sterically demanding Cptt ligand), though the C1-positions on both {Ln(Cptt)2}+ moieties in 4-Ce are always the closest ring carbon atoms to the BH4− units, which contrasts the alternating coordination observed in 1-Ce. This is likely a result of minor steric differences between the Cptt and Htp ligands. The CF splitting of the ground J multiplet is similar in these two systems (626 cm−1 for Ce(1) in 1-Cevs. 642 and 677 cm−1 for 4-Ce; Tables S5, S13 and S14†), therefore, the inclusion of the P heteroatom appears to have minimal effect on the overall CF splitting and ground state stabilisation. However, since the Ce⋯Htp bond lengths are longer, Htp must have a more significant influence on the CF than Cptt in order to arrive at the same magnitude of CF splitting. Whether this is due to P substitution causing an overall increase in ring electron density, or a result of localisation of the electron charge, is unclear.
Conclusions
We have synthesised two families of heteroleptic light Ln3+ complexes with the substituted phospholyl ligand Htp, focusing on Kramers ions and diamagnetic La3+ analogues. In the solid state, the two {Ln(Htp)2}+ fragments of [Ln(Htp)2(μ-BH4)]2 (Ln = La, Ce, Nd, Sm) exhibit significantly different geometries. This is in contrast to the analogous Cptt complexes [Ln(Cptt)2(μ-BH4)]2, showing that sterically similar phospholyl and CpR Ln3+ complexes can show divergent structural behaviour. For the second family of complexes [Ln(Htp)2(μ-BH4)2K(S)2]n, a VT NMR spectroscopic study of the La3+ analogue revealed restricted rotation of the Htp rings in solution. Ab initio calculations of the [Ln(Htp)2(μ-BH4)]2 series showed that there could be dramatic differences in the extent of CF splitting of the two unique Ln3+ sites due to the strong influence of the relative orientation of ring P atoms with Ln3+ centres, but we also observed significant CF effects by variation of equatorial Ln⋯B distances with constant Htp⋯Ln⋯Htp axial fields. Comparisons of the CF splittings in [Ce(Htp)2(μ-BH4)]2 with [Ce(Cptt)2(μ-BH4)]2 indicated that Htp has a relatively large influence on the CF compared to Cptt, which we attribute to either charge localisation or increased ring electron density in Htp due to P substitution. We envisage that such subtle differences in phospholyl and CpR Ln3+ chemistry could be of importance in the construction of geometrically precise f-element complexes, and more specifically for CF engineering in Ln SMM design.Experimental
Materials and methods
All manipulations were carried out using standard Schlenk line and glove box techniques under dry argon. Solvents were passed through columns containing alumina or were dried by refluxing over K, and were stored over K mirrors or 4 Å molecular sieves (THF) and degassed before use. For NMR spectroscopy, C6D6 and C7D8 were dried by refluxing over K. NMR solvents were degassed by three freeze–pump–thaw cycles, and vacuum-transferred before use. [Ln(BH4)3(THF)4] (Ln = La, Ce, Nd, Sm)16 and K(Htp)17 were prepared according to literature methods and KBH4 was used as received. 1H (400 and 500 MHz), 13C{1H} (100 and 125 MHz), 31P{1H} (162 and 202 MHz), and 11B{1H} (128 and 160 MHz) NMR spectra were obtained on Avance III 400 or 500 MHz spectrometers at 298 K. EPR spectroscopy measurements were performed at X-band using a Bruker super-high-Q resonator, and at K-band with a standard cavity, both attached to a Bruker EMX bridge, on powder samples in quartz EPR tubes that were flame-sealed under vacuum. UV-vis-NIR spectroscopy was performed on samples in Youngs tap-appended 10 mm path length quartz cuvettes on an Agilent Technologies Cary Series UV-vis-NIR spectrophotometer at 175–3300 nm. ATR-Fourier transform infrared (ATR-IR) spectra were recorded as microcrystalline powders using a Bruker Tensor 27 spectrometer. Elemental analyses were performed by Mrs Anne Davies and Mr Martin Jennings at The University of Manchester School of Chemistry Microanalysis Service, Manchester, UK. Elemental analysis results for 1-Ln and 2-Ln consistently gave low carbon values, which we assign to carbide formation as other analytical techniques were indicative of bulk purity. General synthetic procedures for 1-Ln, 2-Ln and 3-Ce are given below; full details are in the ESI.†General procedure for synthesis of 1-Ln
Di-n-butyl ether (20 mL) was added to a pre-cooled (−78 °C) Rotaflow tap-appended ampoule containing [Ln(BH4)3(THF)3.5] (2 mmol) and K(Htp) (4 mmol). The reaction mixture was refluxed for 16 hours, allowed to settle and filtered. The solution was concentrated to ca. 2 mL and stored at −25 °C overnight to afford 1-Ln (Ln = La, Ce, Nd, Sm).![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2957 (s), 2900 (m), 2864 (m), 2375 (br, w, B–H str.), 2274 (br, m, B–H str.), 2208 (br, w, B–H str.), 1473 (s), 1460 (s), 1416 (w), 1391 (s), 1359 (s), 1301 (w), 1247 (s), 1194 (s), 1151 (br, m), 1115 (br, s), 1065 (s), 1037 (s), 1020 (w), 991 (s), 920 (w), 888 (w), 817 (s), 794 (s), 720 (s), 660 (s), 611 (s), 590 (s), 517 (w), 429 (w) cm−1.
= 2957 (s), 2900 (m), 2864 (m), 2375 (br, w, B–H str.), 2274 (br, m, B–H str.), 2208 (br, w, B–H str.), 1473 (s), 1460 (s), 1416 (w), 1391 (s), 1359 (s), 1301 (w), 1247 (s), 1194 (s), 1151 (br, m), 1115 (br, s), 1065 (s), 1037 (s), 1020 (w), 991 (s), 920 (w), 888 (w), 817 (s), 794 (s), 720 (s), 660 (s), 611 (s), 590 (s), 517 (w), 429 (w) cm−1.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2948 (br, m), 2872 (br, m), 1644 (m), 1513 (s), 1459 (s), 1383 (br, m), 1276 (m), 1246 (w), 1084 (s), 977 (s), 878 (br, s), 866 (br, s) 820 (w), 796 (w), 774 (s), 740 (s), 705 (s), 683 (s), 661 (s), 636 (s), 611 (m), 554 (w), 521 (w), 422 (w) cm−1.
= 2948 (br, m), 2872 (br, m), 1644 (m), 1513 (s), 1459 (s), 1383 (br, m), 1276 (m), 1246 (w), 1084 (s), 977 (s), 878 (br, s), 866 (br, s) 820 (w), 796 (w), 774 (s), 740 (s), 705 (s), 683 (s), 661 (s), 636 (s), 611 (m), 554 (w), 521 (w), 422 (w) cm−1.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2956 (br, m), 2870 (br, m), 1644 (m), 1513 (s), 1459 (s), 1365 (br, m), 1276 (br, m), 1247 (br, m), 1085 (s), 977 (s), 921 (w), 894 (br, s) 877 (br, s), 865 (br, s), 821 (w), 797 (m), 774 (m), 755 (m), 705 (s), 683 (s), 661 (s), 635 (s), 611 (s), 592 (m), 552 (m), 542 (w), 498 (w), 475 (m), 413 (s) cm−1.
= 2956 (br, m), 2870 (br, m), 1644 (m), 1513 (s), 1459 (s), 1365 (br, m), 1276 (br, m), 1247 (br, m), 1085 (s), 977 (s), 921 (w), 894 (br, s) 877 (br, s), 865 (br, s), 821 (w), 797 (m), 774 (m), 755 (m), 705 (s), 683 (s), 661 (s), 635 (s), 611 (s), 592 (m), 552 (m), 542 (w), 498 (w), 475 (m), 413 (s) cm−1.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2957 (s), 2900 (m), 2865 (m), 2447 (br, m, B–H str.), 2376 (br, w, B–H str.), 2225 (br, s, B–H str.), 1460 (s), 1416 (s), 1391 (s), 1359 (s), 1301 (m), 1248 (s), 1194 (s), 1167 (w), 1085 (br, s), 1065 (s), 1020 (w), 991 (s), 920 (s), 893 (w), 824(w), 799 (s), 759 (s), 721 (m), 694 (s), 661 (s), 611 (s), 591 (s), 517 (w), 464 (w), 435 (w) cm−1.
= 2957 (s), 2900 (m), 2865 (m), 2447 (br, m, B–H str.), 2376 (br, w, B–H str.), 2225 (br, s, B–H str.), 1460 (s), 1416 (s), 1391 (s), 1359 (s), 1301 (m), 1248 (s), 1194 (s), 1167 (w), 1085 (br, s), 1065 (s), 1020 (w), 991 (s), 920 (s), 893 (w), 824(w), 799 (s), 759 (s), 721 (m), 694 (s), 661 (s), 611 (s), 591 (s), 517 (w), 464 (w), 435 (w) cm−1.
General procedure for synthesis of 2-Ln
Dimethoxyethane (20 mL) was added to a pre-cooled (−78 °C) Rotaflow tap-appended ampoule containing [Ln(BH4)3(THF)3.5] (1.5 mmol) and K(Htp) (3.0 mmol). The reaction mixture was allowed to warm to room temperature and refluxed for 16 hours, allowed to settle and filtered. Volatiles were removed in vacuo and toluene (20 mL) was added. The resultant suspension was allowed to settle and filtered. The filtrate was concentrated to 3 mL and stored at −25 °C to afford 2-Ln.![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2946 (s), 2899 (m), 2868 (w), 2427 (s, B–H str.), 2224 (br, m, B–H str.), 2178 (br, m, B–H str.), 1458 (s), 1390 (s), 1363 (s), 1355 (s), 1235 (s), 1152 (s), 1075 (s), 1022 (s), 990 (m), 842 (s), 797 (s), 776 (s), 723 (s), 660 (s), 617 (s), 593 (s) cm−1.
= 2946 (s), 2899 (m), 2868 (w), 2427 (s, B–H str.), 2224 (br, m, B–H str.), 2178 (br, m, B–H str.), 1458 (s), 1390 (s), 1363 (s), 1355 (s), 1235 (s), 1152 (s), 1075 (s), 1022 (s), 990 (m), 842 (s), 797 (s), 776 (s), 723 (s), 660 (s), 617 (s), 593 (s) cm−1.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2945 (s), 2899 (m), 2866 (w), 2828 (w), 2427 (s, B–H str.), 2225 (s, B–H str.), 1457 (s), 1355 (s), 1234 (s), 1194 (s), 1153 (s), 1098 (s), 1076 (s), 1019 (s), 854 (s), 776 (s), 724 (s), 660 (s), 617 (s), 593 (s) cm−1.
= 2945 (s), 2899 (m), 2866 (w), 2828 (w), 2427 (s, B–H str.), 2225 (s, B–H str.), 1457 (s), 1355 (s), 1234 (s), 1194 (s), 1153 (s), 1098 (s), 1076 (s), 1019 (s), 854 (s), 776 (s), 724 (s), 660 (s), 617 (s), 593 (s) cm−1.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2957 (s), 2901 (m), 2866 (m), 2429 (br, w, B–H str.), 2373 (br, w, B–H str.), 2277 (s, B–H str.), 2214 (s, B–H str.), 1460 (s), 1391 (s), 1359 (s), 1248 (s), 1194 (s), 1163 (s), 1115 (s), 1038 (s), 1020 (m), 865 (br, m), 808 (s), 721 (s), 660 (s), 612 (s), 591 (s), 549 (w) cm−1.
= 2957 (s), 2901 (m), 2866 (m), 2429 (br, w, B–H str.), 2373 (br, w, B–H str.), 2277 (s, B–H str.), 2214 (s, B–H str.), 1460 (s), 1391 (s), 1359 (s), 1248 (s), 1194 (s), 1163 (s), 1115 (s), 1038 (s), 1020 (m), 865 (br, m), 808 (s), 721 (s), 660 (s), 612 (s), 591 (s), 549 (w) cm−1.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the China Scholarship Council (studentship for J. L.) the Engineering and Physical Sciences Research Council (EPSRC; EP/P002560/1 for F. O., studentship for L. E. N.), the European Research Council (ERC; Consolidator Grant for D. P. M. and postdoctoral funding for P. J. C. and M. J. G.), The University of Manchester Computational Shared Facility and the EPSRC UK National Electron Paramagnetic Resonance Service for generously supporting this work. We thank Carlo Bawn and Dr Ralph Adams from the NMR Spectroscopy Service for assistance with VT studies. Research data supporting this publication are available from Mendeley Data at DOI: 10.17632/656d55pc7p.1.Notes and references
- Lanthanides: Resource Sustainability, in The Rare Earth Elements: Fundamentals and Applications, ed. D. A. Atwood, Wiley, United Kingdom, 2012 Search PubMed.
- G. Wilkinson and J. M. Birmingham, J. Am. Chem. Soc., 1954, 76, 6210 CrossRef CAS.
- (a) H. Schumann, J. A. Meese-Marktscheffel and L. Esser, Chem. Rev., 1995, 95, 865 CrossRef CAS; (b) O. Walter, Chem. – Eur. J., 2019, 25, 2927 CAS; (c) M. Ephritikhine, Organometallics, 2013, 32, 2464 CrossRef CAS.
- W. J. Evans, Organometallics, 2016, 35, 3088 CrossRef CAS.
- (a) P. Le Floch, Coord. Chem. Rev., 2006, 250, 627 CrossRef CAS; (b) F. Nief, Dalton Trans., 2010, 39, 6589 RSC.
- (a) S. Labouille, F. Nief, X.-F. Le Goff, L. Maron, D. R. Kindra, H. L. Houghton, J. W. Ziller and W. J. Evans, Organometallics, 2012, 31, 5196 CrossRef CAS; (b) H. M. Nicholas and D. P. Mills, in Encyclopedia of Inorganic and Bioinorganic Chemistry, ed. R. A. Scott, John Wiley, Chichester, 2017 Search PubMed.
- F. Nief and F. Mathey, J. Chem. Soc., Chem. Commun., 1989, 800 RSC.
- S. M. Cendrowski-Guillaume, G. L. Gland, M. Nierlich and M. Ephritikhine, Organometallics, 2000, 19, 5654 CrossRef CAS.
- F. Jaroschik, F. Nief, X.-F. Le Goff and L. Ricard, Organometallics, 2007, 26, 3552 CrossRef CAS.
- F. Jaroschik, F. Nief and X.-F. L. Goff, Polyhedron, 2009, 28, 2744 CrossRef CAS.
- H.-J. Gosink, F. Nief, L. Ricard and F. Mathey, Inorg. Chem., 1995, 34, 1306 CrossRef CAS.
- F. Jaroschik, A. Momin, A. Martinez, D. Harakat, L. Ricard, X.-F. Le Goff and G. Nocton, Organometallics, 2016, 35, 2032 CrossRef CAS.
- D. H. Woen, C. M. Kotyk, T. J. Mueller, J. W. Ziller and W. J. Evans, Organometallics, 2017, 36, 4558 CrossRef CAS.
- P. Evans, D. Reta, G. F. S. Whitehead, N. F. Chilton and D. P. Mills, J. Am. Chem. Soc., 2019, 141, 19935 CrossRef CAS PubMed.
- (a) N. A. Pushkarevsky, I. Y. Ilyin, P. A. Petrov, D. G. Samsonenko, M. R. Ryzhikov, P. W. Roesky and S. N. Konchenko, Organometallics, 2017, 36, 1287 CrossRef CAS; (b) C. D. Sofield and R. A. Andersen, J. Organomet. Chem., 1995, 501, 271 CrossRef CAS.
- (a) T. J. Marks and J. R. Kolb, Chem. Rev., 1977, 77, 263 CrossRef CAS; (b) F. Ortu, D. Packer, J. Liu, M. Burton, A. Formanuik and D. P. Mills, J. Organomet. Chem., 2018, 857, 45 CrossRef CAS.
- F. Nief, B. T. de Borms, L. Ricard and D. Carmichael, Eur. J. Inorg. Chem., 2005, 637 CrossRef CAS.
- A. Schnepf, G. Stößer, D. Carmichael, F. Mathey and H. Schnöckel, Angew. Chem., Int. Ed., 1999, 38, 1646 CrossRef CAS PubMed.
- (a) E. B. Lobkovsky, Y. K. Gun'ko, B. M. Bulychev, V. K. Belsky, G. L. Soloveichik and M. Y. Antipin, J. Organomet. Chem., 1991, 406, 343 CrossRef; (b) Y. K. Gun'ko, B. M. Bulychev, G. L. Soloveichik and V. K. Belsky, J. Organomet. Chem., 1992, 424, 289 CrossRef.
- V. A. Volkovich, A. B. Ivanov, S. M. Yakimov, D. V. Tsarevskii, O. A. Golovanova, V. V. Sukhikh and T. R. Griffiths, AIP Conf. Proc., 2016, 1767, 020023-1 CrossRef.
- S. K. Sur, J. Magn. Reson., 1989, 82, 169 CAS.
- O. Kahn, Molecular Magnetism, VCH, Weinheim, 1993 Search PubMed.
- F. Aquilante, J. Autschbach, R. K. Carlson, L. F. Chibotaru, M. G. Delcey, L. De Vico, I. F. Galván, N. Ferré, L. M. Frutos, L. Gagliardi, M. Garavelli, A. Giussani, C. E. Hoyer, G. Li Manni, H. Lischka, D. Ma, P. Å. Malmqvist, T. Müller, A. Nenov, M. Olivucci, T. B. Pedersen, D. Peng, F. Plasser, B. Pritchard, M. Reiher, I. Rivalta, I. Schapiro, J. Segarra-Martí, M. Stenrup, D. G. Truhlar, L. Ungur, A. Valentini, S. Vancoillie, V. Veryazov, V. P. Vysotskiy, O. Weingart, F. Zapata and R. Lindh, J. Comput. Chem., 2016, 37, 506 CrossRef CAS PubMed.
- (a) C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439 CrossRef CAS PubMed; (b) K. R. McClain, C. A. Gould, K. Chakarawet, S. J. Teat, T. J. Groshens, J. R. Long and B. G. Harvey, Chem. Sci., 2018, 9, 8492 RSC; (c) F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400 CrossRef CAS PubMed.
- E. B. Lobkovsky, Y. K. Gun'ko, B. M. Bulychev, V. K. Belsky, G. L. Soloveichik and M. Y. Antipin, J. Organomet. Chem., 1991, 406, 343–352 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1992248–1992254 for 1-Ln, 2-Ln and 3-Ce. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt01241f |
| This journal is © The Royal Society of Chemistry 2020 |