The leaving group in Au(i)–phosphine compounds dictates cytotoxic pathways in CEM leukemia cells and reactivity towards a Cys2His2 model zinc finger†
Abstract
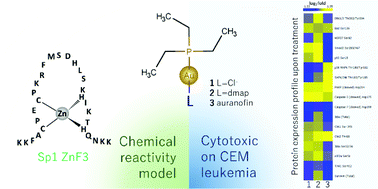
Gold(I)–phosphine “auranofin-like” compounds have been extensively explored as anticancer agents in the past decade. Although potent cytotoxic agents, the lack of selectivity towards tumorigenic vs. non-tumorigenic cell lines often hinders further application. Here we explore the cytotoxic effects of a series of (R3P)AuL compounds, evaluating both the effect of the basicity and bulkiness of the carrier phosphine (R = Et or Cy), and the leaving group L (Cl−vs. dmap). [Au(dmap)(Et3P)]+ had an IC50 of 0.32 μM against the CEM cell line, with good selectivity in relation to HUVEC. Flow cytometry indicates reduced G1 population and slight accumulation in G2, as opposed to auranofin, which induces a high population of cells with fragmented DNA. Protein expression profile sets [Au(dmap)(Et3P)]+ further apart from auranofin, with proteolytic degradation of caspase-3 and poly(ADP-ribose)-polymerase (PARP), DNA strand-break induced phosphorylation of Chk2 Thr68 and increased p53 ser15 phosphorylation. The cytoxicity and observable biological effects correlate directly with the reactivity trend observed when using the series of gold(I)–phosphine compounds for targeting a model zinc finger, Sp1 ZnF3.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...
