Systematically altering the lipophilicity of rhenium(i) tricarbonyl anticancer agents to tune the rate at which they induce cell death†
Abstract
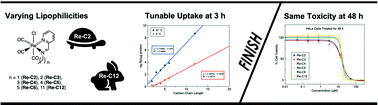
Rhenium-based anticancer agents have arisen as promising alternatives to conventional platinum-based drugs. Based on previous studies demonstrating how increasing lipophilicity improves drug uptake within the cell, we sought to investigate the effects of lipophilicity on the anticancer activity of a series of six rhenium(I) tricarbonyl complexes. These six rhenium(I) tricarbonyl structures, called Re-Chains, bear pyridyl imine ligands with different alkyl chains ranging in length from two to twelve carbons. The cytotoxicities of these compounds were measured in HeLa cells. At long timepoints (48 h), all compounds are equally cytotoxic. At shorter time points, however, the compounds with longer alkyl chains are significantly more active than those with smaller chains. Cellular uptake studies of these compounds show that they are taken up via both passive and active pathways. Collectively, these studies show how lipophilicity affects the rate at which these Re compounds induce their biological activities.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...
