N-heterocyclic carbene-functionalized metal–organic frameworks for the chemical fixation of CO2
Abstract
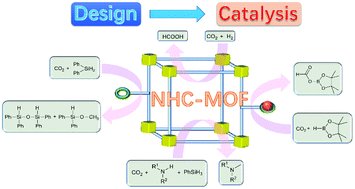
N-heterocyclic carbenes (NHCs) are a class of molecules with a lone pair of carbene electrons and thus, they have the ability to activate CO2 to form imidazolium carboxylates. The incorporation of activated, metal-free NHC moieties into metal–organic frameworks (MOFs) without the decomposition of metal–carboxylate coordination motifs is highly desired owing to the high CO2 affinity and versatile chemical functionalities in MOFs. Herein, we have summarized the recent in situ generation approaches to form metal-free NHC-functionalized MOFs, which are a unique class of CO2-conversion catalysts with high catalytic activity, selectivity and stability, superior to those of homogenous and other heterogeneous NHC analogues. The NHC-functionalized MOFs for catalytic CO2 reduction include reactions such as the hydroboration of CO2, hydrosilylation of CO2, N-methylation using CO2 and hydrogenation of CO2 to formic acid. Overall, the synthetic strategy of metal-free NHC-functionalized MOFs, the unique catalytic pathways of NHC-functionalized MOFs, and potentially new research directions of NHC-functionalized MOFs are discussed, which will guide researchers to attempt to design new NHC-MOFs and extend their catalytic applications in the chemical fixation of CO2.
- This article is part of the themed collection: 2020 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...