Open Access Article
Open Access Article2,5-Bis-trimethylsilyl substituted boroles†
Tobias
Heitkemper
,
Leonard
Naß
and
Christian P.
Sindlinger
 *
*
Institut für Anorganische Chemie, Tammannstr. 4, 37077 Göttingen, Germany. E-mail: christian.sindlinger@chemie.uni-goettingen.de
First published on 6th February 2020
Abstract
This manuscript includes a comprehensive study of the synthesis and spectroscopic features of 2,5-disilyl boroles. Reacting boron trichloride BCl3 with 2,3-Ph*2-1,4-(SiMe3)2-1,4-dilithiobuta-1,3-diene (Ph* = 3,5-t-Bu2(C6H3)) allowed reliable access to 1-Chloro-2,5-(SiMe3)2-3,4-(Ph*)2-borole in good yields (60%). Unlike 2,3,4,5-tetraphenyl haloboroles, this 2,5-bis-trimethylsilyl substituted chloroborole is thermally stable in solution to up to 130 °C. Metathesis reactions of the chloroborole with metal aryls or of the dilithiobutadiene with arylboron dihalides grant access to 1-Ar-2,5-(SiMe3)2-3,4-(Ph*)2 boroles (Ar = Ph, Mes, Ph*, C6F5). Unlike the generally intensely blue-green 2,3,4,5-tetraaryl boroles, brightly orange/red 2,5-bis-trimethylsilyl substituted boroles reveal blue-shifted π/π*-transitions due to a lack of π-system interaction between borole and 2,5-bound aryls. Light is shed on the synthetic peculiarities for the synthesis of 2,5-disilyl-boroles. While direct treatment of the respective 1,1-dimethyl-stannole with ArBCl2via otherwise well-established B/Sn exchange reactions fails, the selectivity of reactions of 2,3-Ph*2-1,4-(SiMe3)2-1,4-dilithiobuta-1,3-diene with ArBCl2 is solvent dependent and leads to rearranged 3-borolenes in hydrocarbons. Gutmann–Beckett analysis reveal reduced Lewis-acidity of disilylboroles compared to pentaphenyl borole.
Introduction
Among unsaturated five-membered main group heterocycles, 1H-boroles adopt a unique position. Isoelectronic to the elusive cyclopentadienyl cation, with four π-electrons in cyclic conjugation via the empty p-orbital of boron, these systems exhibit a weakly anti-aromatic character.1–6 Free boroles are very reactive species that add dihydrogen or silane Si–H bonds across the π-system,7–10 react as potent Lewis-acids also towards weak bases,2,11–13 engage in various Diels–Alder and ring-expansion reactions14–31 or readily accept two electrons to form dianionic 6π-electron systems.32–35 Borolediides are isoelectronic to cyclopentadienyl anions and have thus been used and studied as ligands in transition metal coordination chemistry.32,36–46 Only recently accounts of boroles as π-ligands for p-block elements (Al, Ge) have been reported.47,48 Bearing rather small substituents, free boroles undergo Diels–Alder dimerization. These dimers can provide a source of monomeric borole synthons upon thermal treatment.49–51With free boroles being so reactive, to date, their synthesis and successful isolation is limited to relatively few substituents around the central C4B cycle.52 Most reports on free borole chemistry discuss pentaaryl boroles34,53,54 and particularly (PhC)4BAr for which reliable synthetic protocols exist. Here, a key reaction involves the commercially available diphenyl acetylene which can be readily reductively coupled with lithium to provide 1,4-dilithiobuta-1,3-dienes as valuable precursor for further derivatization.5,6 This allowed for the synthesis of tetraphenylboroles with varying boron-bound substituents in the past.2,33,55–60
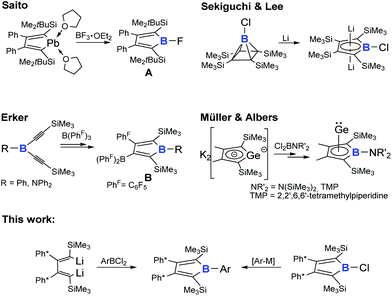
The 2,5-bis-trimethylsilyl-butadiene backbone has recently found application for s-/ and p-block element metaloles, mainly driven by the groups of Xi (groups 2 and 13), Saito and Müller (group 14).61–81 However, only very few examples of 2,5-bis-trialkylsilyl-substituted boroles are known, all of which have been accessed via synthetically rather unusual routes (Scheme 1). Saito and coworkers obtained free fluoroborole Avia Pb/B exchange reactions starting from donor-stabilized plumbole.69 Erker and coworkers reported formation of free 2,5-bis-trimethylsilyl boroles B by 1,1-carboborations after treatment of bis-alkynylboranes with tris(pentafluorophenyl)borane BPhF3.82 Other than these free boroles, the borole motive can be found in Sekiguchi's and Lee's dilithio borolediide obtained from reduction of chloro borapyramidane as well as Müller's and Albers' Ge(II) complex of a borolediide.47,83
We are eager to extend the library of accessible free boroles with regards to the substituents attached to boron and carbon atoms of the central moiety and to study the electronic impacts of each individual substituent.84 Here we report on the synthetic access to 2,5-bis-trimethylsilyl substituted boroles.
Results and discussion
Precursor synthesis
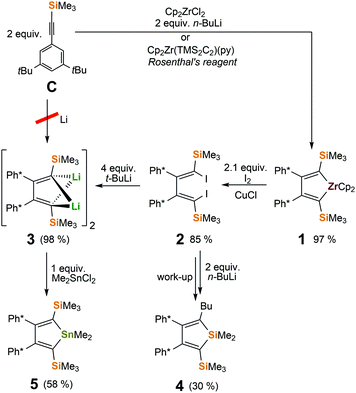
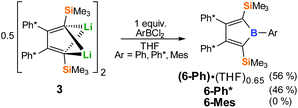
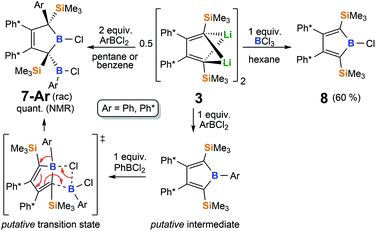
Free boroles (RC)4BR’ are usually accessed via salt metathesis reaction of 1,4-dilithiobuta-1,3-dienes or B/Sn exchange reactions of the respective stannole with boron dihalides RBX2. Stannoles are again either obtained from 1,4-dilithiobuta-1,3-dienes or Fagan–Nugent-type Sn/Zr transmetallations from the respective zirconacyclopentadiene.85–87 1,4-Dilithiobuta-1,3-dienes are accessed by reductive coupling of acetylenes with lithium or from 1,4-diiodobuta-1,3-dienes. The latter are generally derived from zirconacyclopentadienes. However, access to any of these (cyclic) 1,4-dimetalla-1,3-butadiene precursors strongly depends on the nature of the substituents. Especially coupling of silylacetylenes over lithium is heavily depending on the substituents.88We are keen to provide systems that are soluble in apolar hydrocarbon solvents and therefore anticipated 3,5-di-t-butylphenyl-trimethylsilyl acetylene C Ph*CCSiMe3 (Ph* = 3,5-t-Bu2(C6H3)) to be a suitable building block to start from. Acetylene C however did not reveal any reaction with lithium metal and thus we had to take the detour via established zirconacyclopentadiene protocols using Negishi's or Rosenthal's reagents.89,90 2,5-Disilyl-zirconacyclopentadiene 1 forms in excellent yields (>95%) (Scheme 2). However, likely due to steric pressure of the substituents 1 reversibly eliminates alkyne over time (see ESI†).91 Our attempts for direct Zr/Sn transmetallation of 1 with Me2SnCl2 to stannole 5 failed (see ESI†). Reactions of 1 with boron halides RBCl2 neither produced boroles 6 or boryl-borolenes 7 (vide infra) but led to formation of yet unidentified products.
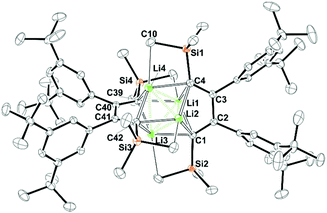
CuCl-supported iodination of in situ generated zirconacyclopentadiene 1 reliably yields the anticipated (Z,Z)-isomer of 1,4-diiodo-butadiene 2.92 In our hands, iodination of previously isolated 1 has under identical conditions led to unfavourable mixtures of (Z,Z) and (E,Z)-isomers of 2 (see ESI†). 2 is conveniently transformed into the 1,4-dilithiobuta-1,3-diene 3 with tert-butyllithium in essentially quantitative yield (98%). 1,4-Dilithiobuta-1,3-diene 3 is obtained as an intensely orange crystalline solid which revealed a dimeric structure in the solid state (Fig. 1). The central distorted [Li4]-tetrahedron reveals its longest Li–Li distances between Li-atoms that are connected to the same butadiene dianion. All lithium atoms feature a further short contact (Li–CTMS 2.423(3)–2.472(3)) to one silicon-bound methyl group thus also providing steric protection of the reactive nucleus. A related structure was previously reported by Saito and coworkers.88
When Li/I exchange was attempted applying n-butyllithium followed by subsequent quenching of in situ generated 3 with Me2SnCl2 at 0 °C, rearrangements were observed to take place and we isolated and identified silole 4 as a major product.93 Under these conditions, butyl iodide apparently reacts with dilithio-butadiene 3. Thus, using t-BuLi to produce 3 remained the more reliable approach. In the presence of HMPA (OP(NMe2)3), formation of 1,1-dimethylsiloles from rearrangements of 1,4-dilithio-1,4-disilylbuta-1,3-dienes, presumably via 2-lithiosilole and MeLi elimination, was described earlier by Xi and coworkers.94,95
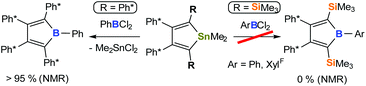
Reaction of 3 with a mild electrophile such as Me2SnCl2 yielded the 1,1-dimethylstannole 5 in moderate crystalline yields (58%).71,96 Unfortunately however, the well-established boron-tin exchange reaction with arylboron dichlorides RBCl2 (R = Ph, XylF {XylF = 3,5-(CF3)2(C6H3)}), that allows convenient and clean access to boroles with 2,3,4,5-tetraaryl-butadiene backbones5,33,84 does not occur in the case of this 2,5-disilyl system (Scheme 3). No formation of Me2SnCl2 is observed in NMR screening reactions. Side reactions leading to intractable product mixtures likely involve methyl abstraction from the tin atom in 5. An abnormal B/Sn exchange reaction behaviour was recently reported by Braunschweig and coworkers.97
Being short of one major synthetic pathway to boroles, we thus turned to direct treatment of 1,4-dilithiobutadiene 3 with organoboron dihalides. Reactions of 3 and 1 equiv. of ArBCl2 in THF revealed to cleanly (>90% by NMR) form the aryl boroles and allowed isolation of 6-Ph and 6-Ph* in good yields (Scheme 4). However, quantitative removal of THF from the resulting Lewis-acidic boroles in vacuo (10−3 mbar) for several days was found to be tedious. The application of THF is therefore usually avoided when free boroles are targeted. While 6-Ph* was obtained free of donor solvent after drying in vacuo, 6-Ph always contained residual amounts of THF, despite their Lewis-acidities were found to be identical (Gutmann–Beckett method, see below). At mildly elevated temperatures (40 °C) THF is liberated from 6-Ph, however substantial decomposition occurs.
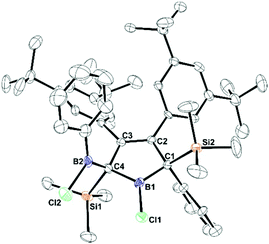
With MesBCl2 the THF route fails completely. In Et2O, borole formation from 3 with ArBCl2 was much less selective and did not provide satisfyingly pure material. However, when 3 was analogously reacted with phenylboron dichloride in hexane or benzene in order to access free organoboroles, irrespective of the applied stoichiometric ratio of the reagents, a clean consumption of 2 equiv. PhBCl2 per 3 took place yielding a 2-borylated 3-borolene 7-Ph. The structure of racemic mixtures of 7-Ph is confirmed by an X-ray structure (Fig. 2) and reveals a double bond between C2 and C3. We reason, that formation of 7-Ph involves the intermediate generation of the anticipated phenyl–borole and rapid subsequent addition of a second equivalent of PhBCl2via an electrophilic attack of PhBCl2 at one Cα atom in the borole (Scheme 5).
These Cα positions should be considerably nucleophilic given they bear two electropositive atom substituents (–SiMe3 and –BR2) and the Si β-effect should further moderate the α-addition of the B-electrophile in these special cases of vinylsilanes. Within the rearrangement sequence, the B-bound Ph-residue then migrates to the other Cα when a chloride adds to the borole B-atom. The addition of a second equivalent ArBCl2 to 6-Ar is facilitated by the removal of anti-aromatic cyclic 4π-electron delocalization and stabilizing lonepair π-donation from Cl into the empty p-orbital of boron. Addition of B–H moieties across a borole to give 2-boryl-3-borolenes where reported earlier.10 Somewhat related chemistry was described for the reaction of azobenzene with (PhC)4BPh.98 Similar reaction behavior consuming 2 equiv. of aryl boron dihalides per 3 are also observed for the reactions with ArBCl2 (Ar = Ph*, XylF). To support the proposed mechanism via formation of the free borole, 1 equiv. of isolated free borole 6-Ph* was treated with the respective Ph*BCl2 to give the same asymmetric compound 2-boryl-3-borolene 7-Ph* as the reaction of 3 with 2 equiv. of Ph*BCl2, thus corroborating the proposed mechanism (see ESI†). Notably, with Ar = Mes (Mes = 2,4,6-Me3(C6H2), no reaction occurred at all, while (PhC)4BMes is reported to be readily formed from (PhC)4Li2 and MesBCl2.58 Also when the electrophilicity of the boron species is reduced such as in i-Pr2NBCl2 or in the Lewis-adduct PhBCl2(DMAP) (DMAP = 4-(N′,N′-dimethylamino)pyridine), dilithio-butadiene 3 did not reveal any reaction in C6H6 or Et2O.
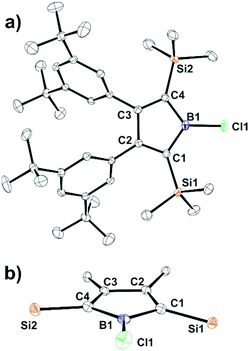
To our surprise, when a solution of 3 was treated with 1 equiv. of boron trichloride in hexane, chloroborole 8 was isolated in moderate yields of about 60% as an orange-red crystalline solid. Unlike in the previously discussed reactions of arylboron dihalides, feasibility of the isolation of 8 likely stems from stabilizing np-π-donation interactions reducing both the Lewis-acidity and anti-aromatic character of chloroborole 8. An X-ray crystallographic examination revealed the structure of 8 as only the third example of a crystallographically characterized haloborole (Fig. 3).
Bond lengths within the C4B-ring are essentially identical to fluoroborole A (Scheme 1) and (PhC)4B–Cl with the B1–Cl1 bond of 1.7543(17) Å in 8 being slightly longer than in the latter (1.7433(13) Å).55 Unlike for these other haloboroles, the substituents around the central C4B-ring slightly bend out of the least-square plane through the borole atoms (Fig. 3b). While substituents at C4 and C3 essentially lie within the plane, substituents at B1, C1 and C2 alternatingly bend out above or below the central plane by ca. 11(1)° each.
The amount of known 1-haloboroles in total is limited to four stable examples. Eisch's and Braunschweig's previously reported 2,3,4,5-tetraphenyl-haloboroles either dimerize at 40 °C ((PhC)4B–Cl)55 or 55 °C ((PhC)4B–Br)57 or even decompose at ambient temperature over time. Marder reported on a transient chloroborole that readily dimerizes.99 This 2,5-disilyl chloroborole 8 is remarkably thermally stable and even forcing conditions in benzene at 130 °C (in a Teflon-valve sealed NMR-tube) for several hours did not indicate any decomposition of 8. Its thermal stability thus more resembles Piers’ perfluorinated 2,3,4,5-tetraphenyl bromoborole (PhFC)4B–Br (PhF = (C6F5)).54 Stable haloboroles are key synthetic precursors to borolyl-substituted systems. By functionalization of (PhC)4BCl, Braunschweig and coworkers previously accessed amino-boroles or even oxidatively added the B–Cl bond across the zero-valent metal in [Pt(PCy3)2].2,56
The 11B-NMR signal of 8 is found as a broad resonance (ω1/2 = 1370 Hz) at δ11B = 70.8 ppm. This is more lowfield shifted than in (PhC)4BCl (δ11B = 66.4 ppm) and (PhFC)4B–Br (δ11B = 67 ppm). Saito's structurally related example of 1-F-2,5-(SiMe2t-Bu)2-3,4-(Ph2)-borole (A), however, features an 11B-NMR resonance at δ11B = 54.9 ppm in line with a pronounced B–F π-interaction.
To explore the synthetic potential of chloroborole 8, we probed the accessibility of boroles by salt-metathesis approaches starting from 8 (Scheme 6). When (Ph*Li)n, (MesLi)2 or Zn(PhF)2 are added to toluene solutions of 8, NMR monitoring indicates the crudes to primarily contain the respective aryl boroles 6-Ar (>80%), however for 6-Ph* and 6-PhF isolated crystalline yields were lower. Notably, attempts to obtain 6-Ph from this approach with PhLi or Ph2Zn in toluene failed and gave intractable product mixtures, that contained intensely colored side products. An intensely green side product (<1% NMR) of unknown constitution was also observed in case of 6-Ph* when prepared via the Ph*Li route. Only reactions of 8 with 0.5 equiv. MgPh2 in THF led to clean formation of 6-Ph which was again contaminated by THF. X-ray structures were obtained for 6-Ar (Ar = Ph*, Mes, PhF) (Fig. 4). Crystals of 6-Ph were repeatedly found to be thin needles that diffracted poorly. Comparable bond lengths within the central C4B ring of all boroles 6-Ar are virtually identical and are well in the range of Erker's related 1-Ph-2,5-disilylborole (B-Ph, Scheme 1)82 and pentaaryl boroles34,54,84 (except for (PhC)4BPh D).1
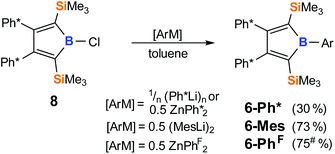 | ||
| Scheme 6 Formation of 6-Ar from 8 (yields after crystallization). #Repeatedly recrystallised product contained ca. 5–10% of an impurity that could not be separated. | ||
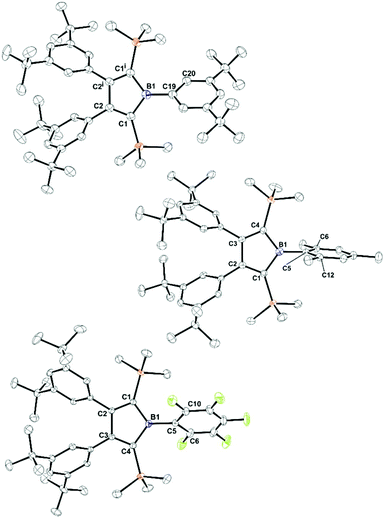 | ||
| Fig. 4 ORTEP plots of the solid-state structure of boroles (6-Ar). Anisotropic displacement parameters are drawn at 50% probability level. Lattice solvent molecules and disordered t-Bu groups are omitted for the sake of clarity. Top: 6-Ph*, middle: 6-Mes, bottom: 6-PhF. Key structural features are summarized in Table 1. | ||
As observed for chloroborole 8, in all cases the silyl-groups, and in some cases but much less pronounced Cβ–Ph*, bend out of the central C4B-plane by 9–15°. Even for aryls (such as Ph or Ph*) that do not feature substituents in ortho-position that would directly govern this torsion angle, the boron-bound aryls reveal rather large torsion angles between the respective C4B- and aryl-planes of >50°. This is likely owing to the bulky silyl groups. With pentaaryl boroles, this torsion angle usually lies between 15–30°. The torsion of the Cβ-bound aryls is much less affected. For 6-Ar these torsions are found between 52–58° not much different from the torsions in (PhC)4BAr (45–55°)1,34 or (Ph*C)4B–Ar (52–58°).84
The 11B-NMR resonances in 6-Ar are found at comparatively low field (Ar = Ph: 76.6 ppm; = Ph*: 77.1 ppm; = Mes: 79.9 ppm; = PhF: 77.4 ppm). Pentaaryl boroles 11B resonances are usually found between 65 and 75 ppm (ref. 1, 30, 54 and 84) and Erker's B-Ph (Scheme 1) at 74.7 ppm.82 Previously, only sterically congested mesityl substituted (ArC)4BMes boroles revealed 11B resonances that low-field shifted (Ar = Ph: 79 ppm; Ar = thienyl: 77 ppm) indicating, that increasing perpendicularity of the B-bound aryl correlates with low-field shifts.
Properties of 2,5-disilylboroles
NICS101–103 values for 6-Ar and 8 were calculated and NICS(0) and NICS(1) are tabulated in Table 1. The impact of the 2,5-disilyl substitution pattern on the (anti)aromaticity of boroles causes the NICS(0) values to be lower than the parent E (20.6), but higher (with a maximum of 16.7 for 6-PhF) than D (PhC)4BPh (13.6). However the NICS-profiles of 6-Ar drop steeper and NICS(1) values do not differ significantly from D (see ESI†). The Lewis acidity of 2,5-disilylboroles was probed by means of the Gutmann–Beckett approach that correlates the 31P chemical shift of Et3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interacting with Lewis acids with their acidity.104–106 For steric reasons the Lewis-acidity of 6-Mes is poorly accounted for by this method but 8 (70.7), 6-Ph* (72.1), 6-Ph (72.1) and even 6-PhF (73.8) all reveal similar acceptor numbers (AN) well below those previously determined under identical conditions for D (78.7).100 The bulky and electropositive silyl groups seem to lower the Lewis-acidity, however the method cannot provide insight which influence is dominating.
O interacting with Lewis acids with their acidity.104–106 For steric reasons the Lewis-acidity of 6-Mes is poorly accounted for by this method but 8 (70.7), 6-Ph* (72.1), 6-Ph (72.1) and even 6-PhF (73.8) all reveal similar acceptor numbers (AN) well below those previously determined under identical conditions for D (78.7).100 The bulky and electropositive silyl groups seem to lower the Lewis-acidity, however the method cannot provide insight which influence is dominating.
| Entry | B–Cα, Cα–Cβ, Cβ–Cβ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
C4B–Ar torsionb | δ 13C (Cα, Cβ)c | δ 11Bc | λ exp,d (λcalc),eελf | HOMO/LUMO/gapg | NICSh | δ 31Pi AN |
|---|---|---|---|---|---|---|---|---|
| a In [Å]. b Torsion angle in [°]. c In parts per million, ppm. d Absorption bands of lowest energy in nm. e TD-DFT: RIJCOSX-CAM-B3LYP\def2-SVP\\RI-BP86-D3BJ\def2-TZVP. f In L mol−1 cm−1. g RI-BP86\def2-TZVP, energies in eV. h NICSiso(0) and NICSiso(1) GIAO PBE0\def2-TZVP. i Gutmann–Beckett Lewis-acidity scale parameters derived from mixtures with Et3PO in C6D6. Acceptor Numbers AN = 2.21 × (δ31P −41). j Bond lengths as reported in ref. 1. Note that C4B bond lengths in this structure markedly deviate from other known pentaaryl boroles. | ||||||||
| 8 chloroborole | 1.568(2), 1.353(2), 1.538(2) | — | 134.8, 182.1 | 70.8 | 447, (450) | −5.33/−3.55 | 14.0 | 73.0 |
| 1.564(2), 1.354(2) | 258 | 1.78 | 6.5 | 70.7 | ||||
| 6-Ph | — | — | 139.2, 183.1 | 76.6 | 480, (475) | −5.16/−3.50 | 15.1 | 73.6 |
| — | 1.66 | 7.4 | 72.1 | |||||
| 6-Ph* | 1.587(3), 1.355(3), 1.540(4) | 51 | 139.2, 182.8 | 77.1 | 473, (469) | −5.11/−3.41 | 14.7 | 73.6 |
| 130 | 1.70 | 7.1 | 72.1 | |||||
| 6-Mes | 1.590(3), 1.360(4), 1.538(4) | 85 | 138.8, 181.4 | 79.9 | ≈480, (462) | −5.17/−3.45 | 14.6 | 46.1 |
| 1.594(4), 1.359(3) | 310 | 1.72 | 6.8 | 11.3 | ||||
| 6-PhF | 1.577(2), 1.359(2), 1.539 (2) | 59 | 137.6, 184.8 | 77.4 | ≈505, (510) | −5.37/−3.83 | 16.7 | 74.4 |
| 1.577(2), 1.357(2) | 121 | 1.54 | 8.4 | 73.8 | ||||
| D1 (PhC)4BPh | 1.526(2), 1.428(2), 1.470(2) | 33 | 137.9, 162.1 | 65.4 | 560, (577) | −4.84/−3.64 | 13.6 | 76.6100 |
| 1.539(2), 1.425(2)j | 1.20 | 7.2 | 78.7 | |||||
| E (HC)4BH | 1.585, 1.348, 1.520g | — | — | — | —, (466) | −5.72/−3.95 | 20.6 | — |
| 1.77 | 11.5 | |||||||
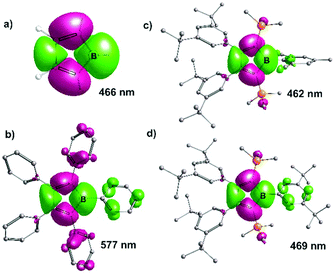
Since the isolation of deeply-blue (PhC)4BPh (λmax 560 nm),5 a striking feature of the hitherto well-characterized examples of pentaaryl boroles is their intense color with broad absorptions in the visible spectra roughly spanning from λmax 530 nm (purple (PhFC)4BPhF)54 to 635 nm (green (PhC)4BPhF).30 Substitution with heteroaryls (e.g. thiophene) allowed even stronger shifts of λmax.53,60 The 2,5-disilyl-substituted boroles reported here are all orange to red both in solid state and in solution with absorption bands around 470 nm. We originally anticipated that having electropositive substituents, such as the SiMe3 group, attached to the Cα-carbons in boroles should narrow the π/π* gap, which is dominatingly responsible for the intense colorization, and thus induce a red-shifted absorption. However, the opposite seems to be the case. We therefore computationally probed the photochemical properties of the elusive parent borole (HC)4BH (E), the 2,5-disilyl-boroles 6-Ar and pentaphenyl borole (PhC)4BPh (D) (Fig. 5).
TD-DFT calculations on 8 and 6-Ar reproduced the experimental absorption spectra very well. In all cases the lowest energy absorption is dominated by the π/π* transition between the borole C4B based HOMO and LUMO. The absorption for the π/π* transition in parent (HC)4BH E is predicted at 466 nm well in the range where the respective resonances for 6-Ar are found. The difference density plots for the respective excitations of E and 6-Mes are very similar and highlight only minor contributions from substituents (Fig. 5).
Pentaphenylborole (PhC)4BPh however reveals major contributions both from the boron-bound (accepting) and Cα-bound (donating) phenyl π-systems. From comparison with our 2,5-disilylboroles we reason, that particularly the π-interaction of C4B with Cα-bound aryls causes the red-shifted absorption that leads to the intense purple to green colors of (substituted) pentaphenyl boroles. Thus the 2,5-disilylboroles without perturbation of the C4B π-system by arene ligands represent a much more accurate synthetic model of the parent, but elusive, (HC)4BH E when it comes to direct comparisons of the frontier orbital situation. This is also reflected in the HOMO–LUMO energies (Table 1).
To shed further light on the influence of aryl π-system interaction in boroles, in a qualitative computational approach, we probed the individual influence of C4B bound aryls on the computationally predicted π/π* absorption band (Fig. 6).
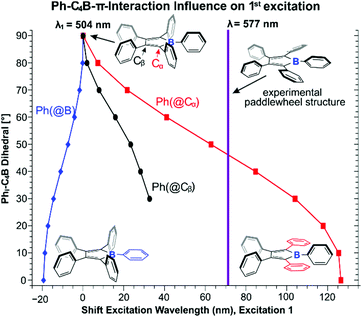 | ||
| Fig. 6 TD-DFT predicted shifts in excitation wavelength of (PhC)4BPh depending on the torsion angle between C4B-plane and B-bound, Cα-bound or Cβ-bound phenyls. | ||
Starting from a hypothetical structure of (PhC)4BPh with all phenyl groups perpendicular to the C4B plane, TD-DFT predicts the absorption at 504 nm. Minimizing effective π-interaction thus apparently results in blueshifted absorptions closer to unperturbed (HC)4BH (E). From there on, gradually reducing the (C4B)–Phi torsion angle at B-, Cα- and (to a reduced extent due to eventual collapsing overlap) Cβ-bound aryls to coplanarity allows a qualitative insight into the individual effects. While co-planarily bound B–Ph groups lead to only mildly blue-shifted absorptions likely due to a LUMO raise on account of more effective π-interaction, particularly co-planarily arranged Cα-bound phenyls reveal a strong red-shifting effect. This further corroborates the essential impact of Cα-bound aryls on the color and HOMO/LUMO gap of boroles that we identified from their substitution by silyl groups. The vast influence of the Cα aryl torsion may also be a reason for the broad bands (ω1/2 ≈ 200 nm) commonly observed for these transitions in pentaphenyl boroles. Some previous studies on heteroaryl substituted boroles already suggested the importance of torsion-angle dependent π-interaction affecting the spectroscopic features.53,60
Conclusion
In summary we presented various synthetic approaches to 2,5-disilyl boroles and shed light on their limitations. While traditional routes via boron-tin exchange reactions from stannoles fail, the reaction of the 1,4-dilithiobutadiene with aryl borondichlorides provides access to substituted boroles when the reaction is conducted in THF. In hydrocarbons two equivalents of arylboron dihalides react with 1,4-dilithiobutadienes to afford 2-boryl-3-borolenes, putatively via the free borole. Treatment of 1,4-dilithiobutadiene with BCl3 grants access to a thermally robust chloroborole. The reactions of the chloroborole with common available aryl–carbon nucleophiles such as aryllithium, Grignard reagents and arylzinc reagents revealed to be very dependent on the substituent and often leads to colored product mixtures.π/π*-absorption features of 2,5-disilyl-boroles are distinctive from pentaaryl boroles in that they do not reveal deeply blue colors. The differences were routed back to absence of π-interaction contributions stemming from Cα-bound aryl groups that are the foundation of the purple-to-blue color of pentaaryl boroles. Spectroscopically and regarding the frontier orbital situation, 2,5-disilylboroles are much closer to the parent, yet elusive borole (HC)4BH. Given the field of free borole chemistry has been dominated by tetraphenyl-butadiene systems for 50 years, the new boroles and their access routes reported in this contribution represent a significant extension to the existing library of substitution patterns that allow the handling of free boroles. We are currently exploring the chemical potential of these boroles.
Experimental
Extensive synthetic and analytical details are given in the ESI.†Conflicts of interest
Content of this manuscript is or will be included in the PhD/BSc theses of T. H. and L. N. There are no conflicts to declare.Acknowledgements
Prof. Dietmar Stalke is most gratefully acknowledged for generous and supportive mentorship. We are grateful to the Fonds der chemischen Industrie for funding (fellowships to CPS and TH). The institute of inorganic chemistry is thanked for the provision of excellent conditions. We are grateful to LANXESS Organometallics GmbH for a chemical donation. MSc Paul Niklas Ruth is acknowledged for crystallographic advice and expertise. This contribution was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 389479699/GRK2455.Notes and references
- H. Braunschweig, I. Fernández, G. Frenking and T. Kupfer, Angew. Chem., Int. Ed., 2008, 47, 1951–1954 CrossRef CAS PubMed.
- H. Braunschweig and T. Kupfer, Chem. Commun., 2008, 37, 4487–4489 RSC.
- H. Braunschweig, I. Krummenacher and J. Wahler, in Advances in Organometallic Chemistry, ed. A. F. Hill and M. J. Fink, Academic Press, 2013, vol. 61, pp. 1–53 Search PubMed.
- J. O. C. Jimenez-Halla, E. Matito, M. Sola, H. Braunschweig, C. Horl, I. Krummenacher and J. Wahler, Dalton Trans., 2015, 44, 6740–6747 RSC.
- J. J. Eisch, N. K. Hota and S. Kozima, J. Am. Chem. Soc., 1969, 91, 4575–4577 CrossRef CAS.
- J. J. Eisch, J. E. Galle and S. Kozima, J. Am. Chem. Soc., 1986, 108, 379–385 CrossRef CAS PubMed.
- C. Fan, L. G. Mercier, W. E. Piers, H. M. Tuononen and M. Parvez, J. Am. Chem. Soc., 2010, 132, 9604–9606 CrossRef CAS PubMed.
- A. Y. Houghton, V. A. Karttunen, C. Fan, W. E. Piers and H. M. Tuononen, J. Am. Chem. Soc., 2013, 135, 941–947 CrossRef CAS PubMed.
- H. Braunschweig, A. Damme, C. Hörl, T. Kupfer and J. Wahler, Organometallics, 2013, 32, 6800–6803 CrossRef CAS.
- B. C. Caputo, Z. J. Manning, J. H. Barnard and C. D. Martin, Polyhedron, 2016, 114, 273–277 CrossRef CAS.
- A. Fukazawa, J. L. Dutton, C. Fan, L. G. Mercier, A. Y. Houghton, Q. Wu, W. E. Piers and M. Parvez, Chem. Sci., 2012, 3, 1814–1818 RSC.
- H. Braunschweig, C.-W. Chiu, T. Kupfer and K. Radacki, Inorg. Chem., 2011, 50, 4247–4249 CrossRef CAS PubMed.
- K. Ansorg, H. Braunschweig, C. W. Chiu, B. Engels, D. Gamon, M. Hügel, T. Kupfer and K. Radacki, Angew. Chem., Int. Ed., 2011, 50, 2833–2836 CrossRef CAS PubMed.
- J. J. Eisch and J. E. Galle, J. Am. Chem. Soc., 1975, 97, 4436–4437 CrossRef CAS.
- J. J. Eisch, J. E. Galle, B. Shafii and A. L. Rheingold, Organometallics, 1990, 9, 2342–2349 CrossRef CAS.
- S. A. Couchman, T. K. Thompson, D. J. D. Wilson, J. L. Dutton and C. D. Martin, Chem. Commun., 2014, 50, 11724–11726 RSC.
- K. Huang, S. A. Couchman, D. J. D. Wilson, J. L. Dutton and C. D. Martin, Inorg. Chem., 2015, 54, 8957–8968 CrossRef CAS PubMed.
- K. Huang and C. D. Martin, Inorg. Chem., 2015, 54, 1869–1875 CrossRef CAS PubMed.
- J. H. Barnard, S. Yruegas, K. Huang and C. D. Martin, Chem. Commun., 2016, 52, 9985–9991 RSC.
- S. Yruegas, D. C. Patterson and C. D. Martin, Chem. Commun., 2016, 52, 6658–6661 RSC.
- K. Huang and C. D. Martin, Inorg. Chem., 2016, 55, 330–337 CrossRef CAS PubMed.
- J. H. Barnard, S. Yruegas, S. A. Couchman, D. J. D. Wilson, J. L. Dutton and C. D. Martin, Organometallics, 2016, 35, 929–931 CrossRef CAS.
- S. Yruegas and C. D. Martin, Chem. – Eur. J., 2016, 22, 18358–18361 CrossRef CAS PubMed.
- S. Yruegas, C. Wilson, J. L. Dutton and C. D. Martin, Organometallics, 2017, 36, 2581–2587 CrossRef CAS.
- J. J. Baker, K. H. M. Al-Furaiji, O. T. Liyanage, D. J. D. Wilson, J. L. Dutton and C. D. Martin, Chem. – Eur. J., 2019, 25, 1581–1587 CrossRef CAS PubMed.
- H. Braunschweig, C. Hörl, L. Mailänder, K. Radacki and J. Wahler, Chem. – Eur. J., 2014, 20, 9858–9861 CrossRef CAS PubMed.
- H. Braunschweig, F. Hupp, I. Krummenacher, L. Mailänder and F. Rauch, Chem. – Eur. J., 2015, 21, 17844–17849 CrossRef CAS PubMed.
- H. Braunschweig, I. Krummenacher, L. Mailänder and F. Rauch, Chem. Commun., 2015, 51, 14513–14515 RSC.
- H. Braunschweig, M. A. Celik, F. Hupp, I. Krummenacher and L. Mailänder, Angew. Chem., Int. Ed., 2015, 54, 6347–6351 CrossRef CAS PubMed.
- H. Braunschweig, M. A. Celik, T. Dellermann, G. Frenking, K. Hammond, F. Hupp, H. Kelch, I. Krummenacher, F. Lindl, L. Mailänder, J. H. Müssig and A. Ruppert, Chem. – Eur. J., 2017, 23, 8006–8013 CrossRef CAS PubMed.
- F. Lindl, S. Lin, I. Krummenacher, C. Lenczyk, A. Stoy, M. Müller, Z. Lin and H. Braunschweig, Angew. Chem., Int. Ed., 2019, 58, 338–342 CrossRef CAS PubMed.
- G. E. Herberich, B. Buller, B. Hessner and W. Oschmann, J. Organomet. Chem., 1980, 195, 253–259 CrossRef CAS.
- H. Braunschweig, C.-W. Chiu, A. Damme, B. Engels, D. Gamon, C. Hörl, T. Kupfer, I. Krummenacher, K. Radacki and C. Walter, Chem. – Eur. J., 2012, 18, 14292–14304 CrossRef CAS PubMed.
- C.-W. So, D. Watanabe, A. Wakamiya and S. Yamaguchi, Organometallics, 2008, 27, 3496–3501 CrossRef CAS.
- H. Braunschweig and I. Krummenacher, in Organic Redox Systems, 2015, pp. 503–522, DOI:10.1002/9781118858981.ch17.
- G. E. Herberich, J. Hengesbach, U. Kölle, G. Huttner and A. Frank, Angew. Chem., Int. Ed. Engl., 1976, 15, 433–434 CrossRef.
- G. E. Herberich, J. Hengesbach, U. Kölle and W. Oschmann, Angew. Chem., Int. Ed. Engl., 1977, 16, 42–43 CrossRef.
- G. E. Herberich, B. Hessner, W. Boveleth, H. Lüthe, R. Saive and L. Zelenka, Angew. Chem., Int. Ed. Engl., 1983, 22, 996–996 CrossRef.
- G. E. Herberich, W. Boveleth, B. Hessner, D. P. J. Köffer, M. Negele and R. Saive, J. Organomet. Chem., 1986, 308, 153–166 CrossRef CAS.
- G. E. Herberich, B. Hessner, H. Ohst and I. A. Raap, J. Organomet. Chem., 1988, 348, 305–316 CrossRef CAS.
- G. E. Herberich, U. Englert, M. Hostalek and R. Laven, Chem. Ber., 1991, 124, 17–23 CrossRef CAS.
- G. E. Herberich, T. Carstensen and U. Englert, Chem. Ber., 1992, 125, 2351–2357 CrossRef CAS.
- R. W. Quan, G. C. Bazan, W. P. Schaefer, J. E. Bercaw and A. F. Kiely, J. Am. Chem. Soc., 1994, 116, 4489–4490 CrossRef CAS.
- C. K. Sperry, W. D. Cotter, R. A. Lee, R. J. Lachicotte and G. C. Bazan, J. Am. Chem. Soc., 1998, 120, 7791–7805 CrossRef CAS.
- G. J. Pindado, S. J. Lancaster, M. Thornton-Pett and M. Bochmann, J. Am. Chem. Soc., 1998, 120, 6816–6817 CrossRef CAS.
- T. J. Woodman, M. Thornton-Pett, D. L. Hughes and M. Bochmann, Organometallics, 2001, 20, 4080–4091 CrossRef CAS.
- P. Tholen, Z. Dong, M. Schmidtmann, L. Albers and T. Müller, Angew. Chem., Int. Ed., 2018, 57, 13319–13324 CrossRef CAS PubMed.
- C. P. Sindlinger and P. N. Ruth, Angew. Chem., Int. Ed., 2019, 58, 15051–15056 CrossRef CAS PubMed.
- P. J. Fagan, E. G. Burns and J. C. Calabrese, J. Am. Chem. Soc., 1988, 110, 2979–2981 CrossRef CAS.
- X. Su, J. J. Baker and C. D. Martin, Chem. Sci., 2020, 11, 126–131 RSC.
- J. J. Baker, K. H. M. Al Furaiji, O. T. Liyanage, D. J. D. Wilson, J. L. Dutton and C. D. Martin, Chem. – Eur. J., 2019, 25, 1581–1587 CrossRef CAS PubMed.
- This discussion does not include systems where the borole 4π-electron system is embedded in larger, delocalised aromatic framworks as for example in 9-borafluorenes.
- T. Araki, A. Fukazawa and S. Yamaguchi, Angew. Chem., Int. Ed., 2012, 51, 5484–5487 CrossRef CAS PubMed.
- C. Fan, W. E. Piers and M. Parvez, Angew. Chem., Int. Ed., 2009, 48, 2955–2958 CrossRef CAS PubMed.
- H. Braunschweig, C.-W. Chiu, J. Wahler, K. Radacki and T. Kupfer, Chem. – Eur. J., 2010, 16, 12229–12233 CrossRef CAS PubMed.
- H. Braunschweig, C.-W. Chiu, K. Radacki and P. Brenner, Chem. Commun., 2010, 46, 916–918 RSC.
- H. Braunschweig, C.-W. Chiu, A. Damme, K. Ferkinghoff, K. Kraft, K. Radacki and J. Wahler, Organometallics, 2011, 30, 3210–3216 CrossRef CAS.
- H. Braunschweig, V. Dyakonov, J. O. C. Jimenez-Halla, K. Kraft, I. Krummenacher, K. Radacki, A. Sperlich and J. Wahler, Angew. Chem., Int. Ed., 2012, 51, 2977–2980 CrossRef CAS PubMed.
- H. Braunschweig, C.-W. Chiu, D. Gamon, M. Kaupp, I. Krummenacher, T. Kupfer, R. Müller and K. Radacki, Chem. – Eur. J., 2012, 18, 11732–11746 CrossRef CAS PubMed.
- H. Braunschweig, A. Damme, J. O. C. Jimenez-Halla, C. Hörl, I. Krummenacher, T. Kupfer, L. Mailänder and K. Radacki, J. Am. Chem. Soc., 2012, 134, 20169–20177 CrossRef CAS PubMed.
- A. J. Ashe, J. W. Kampf and P. M. Savla, Organometallics, 1993, 12, 3350–3353 CrossRef CAS.
- L. Liu, W.-X. Zhang, Q. Luo, H. Li and Z. Xi, Organometallics, 2010, 29, 278–281 CrossRef CAS.
- S. Zhang, M. Zhan, W.-X. Zhang and Z. Xi, Organometallics, 2013, 32, 4020–4023 CrossRef CAS.
- J. Wei, L. Liu, M. Zhan, L. Xu, W.-X. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2014, 53, 5634–5638 CrossRef CAS PubMed.
- Y. Zhang, J. Wei, W.-X. Zhang and Z. Xi, Inorg. Chem., 2015, 54, 10695–10700 CrossRef CAS PubMed.
- Y. Zhang, Y. Chi, J. Wei, Q. Yang, Z. Yang, H. Chen, R. Yang, W.-X. Zhang and Z. Xi, Organometallics, 2017, 36, 2982–2986 CrossRef CAS.
- B. Wei, L. Liu, W.-X. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2017, 56, 9188–9192 CrossRef CAS PubMed.
- Y. Zhang, Z. Yang, W.-X. Zhang and Z. Xi, Chem. – Eur. J., 2019, 25, 4218–4224 CrossRef CAS PubMed.
- M. Saito, T. Akiba, M. Kaneko, T. Kawamura, M. Abe, M. Hada and M. Minoura, Chem. – Eur. J., 2013, 19, 16946–16953 CrossRef CAS PubMed.
- T. Kuwabara, J.-D. Guo, S. Nagase, T. Sasamori, N. Tokitoh and M. Saito, J. Am. Chem. Soc., 2014, 136, 13059–13064 CrossRef CAS PubMed.
- T. Kuwabara, J.-D. Guo, S. Nagase, M. Minoura, R. H. Herber and M. Saito, Organometallics, 2014, 33, 2910–2913 CrossRef CAS.
- T. Kuwabara and M. Saito, Organometallics, 2015, 34, 4202–4204 CrossRef CAS.
- M. Nakada, T. Kuwabara, S. Furukawa, M. Hada, M. Minoura and M. Saito, Chem. Sci., 2017, 8, 3092–3097 RSC.
- M. Saito, T. Akiba, S. Furukawa, M. Minoura, M. Hada and H. Y. Yoshikawa, Organometallics, 2017, 36, 2487–2490 CrossRef CAS.
- Z. Dong, C. R. W. Reinhold, M. Schmidtmann and T. Müller, J. Am. Chem. Soc., 2017, 139, 7117–7123 CrossRef CAS PubMed.
- Z. Dong, O. Janka, J. Kösters, M. Schmidtmann and T. Müller, Angew. Chem., Int. Ed., 2018, 57, 8634–8638 CrossRef CAS PubMed.
- Z. Dong, C. R. W. Reinhold, M. Schmidtmann and T. Müller, Organometallics, 2018, 37, 4736–4743 CrossRef CAS.
- Z. Dong, M. Schmidtmann and T. Müller, Z. Anorg. Allg. Chem., 2018, 644, 1041–1046 CrossRef CAS.
- C. R. W. Reinhold, Z. Dong, J. M. Winkler, H. Steinert, M. Schmidtmann and T. Müller, Chem. – Eur. J., 2018, 24, 848–854 CrossRef CAS.
- Z. Dong, M. Schmidtmann and T. Müller, Chem. – Eur. J., 2019, 25, 10858–10865 CrossRef CAS PubMed.
- Z. Dong, L. Albers, M. Schmidtmann and T. Müller, Chem. – Eur. J., 2019, 25, 1098–1105 CrossRef CAS.
- F. Ge, G. Kehr, C. G. Daniliuc and G. Erker, J. Am. Chem. Soc., 2014, 136, 68–71 CrossRef CAS PubMed.
- V. Y. Lee, H. Sugasawa, O. A. Gapurenko, R. M. Minyaev, V. I. Minkin, H. Gornitzka and A. Sekiguchi, J. Am. Chem. Soc., 2018, 140, 6053–6056 CrossRef CAS PubMed.
- T. Heitkemper and C. P. Sindlinger, Chem. – Eur. J., 2019, 25, 6628–6637 CrossRef CAS PubMed.
- X. Yan and C. Xi, Acc. Chem. Res., 2015, 48, 935–946 CrossRef CAS PubMed.
- P. J. Fagan and W. A. Nugent, J. Am. Chem. Soc., 1988, 110, 2310–2312 CrossRef CAS.
- P. J. Fagan, W. A. Nugent and J. C. Calabrese, J. Am. Chem. Soc., 1994, 116, 1880–1889 CrossRef CAS.
- M. Saito, M. Nakamura, T. Tajima and M. Yoshioka, Angew. Chem., Int. Ed., 2007, 46, 1504–1507 CrossRef CAS PubMed.
- E.-I. Negishi and T. Takahashi, Acc. Chem. Res., 1994, 27, 124–130 CrossRef CAS.
- U. Rosenthal, A. Ohff, W. Baumann, A. Tillack, H. Görls, V. V. Burlakov and V. B. Shur, Z. Anorg. Allg. Chem., 1995, 621, 77–83 CrossRef CAS.
- A. D. Miller, J. F. Tannaci, S. A. Johnson, H. Lee, J. L. McBee and T. D. Tilley, J. Am. Chem. Soc., 2009, 131, 4917–4927 CrossRef CAS PubMed.
- C. Xi, S. Huo, T. H. Afifi, R. Hara and T. Takahashi, Tetrahedron Lett., 1997, 38, 4099–4102 CrossRef CAS.
- The crystal structure of 4 is documented in the ESI.†.
- C. Wang, Q. Luo, H. Sun, X. Guo and Z. Xi, J. Am. Chem. Soc., 2007, 129, 3094–3095 CrossRef CAS PubMed.
- Q. Luo, C. Wang, L. Gu, W.-X. Zhang and Z. Xi, Chem. – Asian J., 2010, 5, 1120–1128 CrossRef CAS PubMed.
- Analytical and crystallographic data for 5 is documented in the ESI.†.
- H. Braunschweig, M. Dömling, S. Kachel, H. Kelch, T. Kramer, I. Krummenacher, C. Lenczyk, S. Lin, Z. Lin, C. Possiel and K. Radacki, Chem. – Eur. J., 2017, 23, 16167–16170 CrossRef CAS PubMed.
- V. A. K. Adiraju and C. D. Martin, Chem. – Eur. J., 2017, 23, 11437–11444 CrossRef CAS PubMed.
- Z. Zhang, Z. Wang, M. Haehnel, A. Eichhorn, R. M. Edkins, A. Steffen, A. Krueger, Z. Lin and T. B. Marder, Chem. Commun., 2016, 52, 9707–9710 RSC.
- S. Yruegas, J. J. Martinez and C. D. Martin, Chem. Commun., 2018, 54, 6808–6811 RSC.
- P. V. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. van Eikema Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. V. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- A. Stanger, J. Org. Chem., 2006, 71, 883–893 CrossRef CAS PubMed.
- U. Mayer, V. Gutmann and W. Gerger, Monatsh. Chem., 1975, 106, 1235–1257 CrossRef CAS.
- M. A. Beckett, G. C. Strickland, J. R. Holland and K. Sukumar Varma, Polymer, 1996, 37, 4629–4631 CrossRef CAS.
- I. B. Sivaev and V. I. Bregadze, Coord. Chem. Rev., 2014, 270–271, 75–88 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and synthetic details, depiction of spectra and crystal structures, computational details. CCDC 1976852–1976861. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt00393j |
| This journal is © The Royal Society of Chemistry 2020 |