Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of magnetic field on the hydrogen evolution activity using non-magnetic Weyl semimetal catalysts†
Uttam
Gupta
 ab,
Catherine R.
Rajamathi
a,
Nitesh
Kumar
ab,
Catherine R.
Rajamathi
a,
Nitesh
Kumar
 *a,
Guowei
Li
*a,
Guowei
Li
 a,
Yan
Sun
a,
Yan
Sun
 a,
Chandra
Shekhar
a,
Chandra
Shekhar
 a,
Claudia
Felser
a,
Claudia
Felser
 *a and
C. N. R.
Rao
*a and
C. N. R.
Rao
 *b
*b
aMax Planck Institute for Chemical Physics of Solids, 01187 Dresden, Germany. E-mail: Kumar@cpfs.mpg.de; Felser@cpfs.mpg.de
bNew Chemistry Unit, International Centre for Materials Science, Sheikh Saqr Laboratory, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur P.O., Bangalore-560064, India. E-mail: cnrrao@jncasr.ac.in
First published on 21st February 2020
Abstract
An external switch to control the kinetics of the reaction by manipulating the participating electrons could be interesting as it can alter the rate of the reaction without affecting the reaction pathway. The magnetic field, like a switch, is non-invasive, tunable, and clean; it can also alter the electrons in a material. We study the effect of an applied magnetic field on the hydrogen evolution activity of the NbP family of Weyl semimetals because of their extremely high mobility and large magnetoresistance at room temperature and good hydrogen evolution properties. We find that by applying a magnetic field of ∼3500 G, the hydrogen evolution activity of NbP increases by up to 95%. The other members of this Weyl semimetal family (viz. TaP, NbAs, and TaAs) also exhibit increased hydrogen evolution activity. Thus, our observations suggest an interplay of electronic property, magnetic field, and catalytic activity in this class of compounds, providing evidence of manipulating the catalytic performance of topological materials through the application of a magnetic field.
Heterogeneous catalytic reactions such as hydrogen evolution are associated with the surface electronic states or surface atomic termination of catalysts.1,2 Catalysts with high carrier mobility and low hydrogen binding energy are desirable. For instance, two different polymorphs of molybdenum disulfide, namely semiconducting 2H-MoS2 and metallic 1T-MoS2, exhibit contrasting hydrogen evolution properties. The 1T-form has better conductivity which leads to a considerable hydrogen evolution reaction (HER) activity.3–5 In general, 2D-structures with high in-plane conductivity and exposed surface exhibit excellent catalytic activity.6,7 They also provide templates to grow other materials, which further enhances the overall activity of catalysts.8,9 For example, the importance of the mobility of electrons in hydrogen evolution catalysts had also been proved earlier by using graphene as a medium for efficient transport of charge carriers, wherein linearly dispersing bands near the Dirac points of graphene are the source of the high mobility of charge carriers.10,11 Topological materials, such as topological insulators, Weyl semimetals or nodal line semimetals, are robust against surface modifications and defects derived from dangling bonds, vacancies, or doping which otherwise destroy surface states.12–15 Topological surface states (TSSs) are a result of the inversion of bulk bands, with the electron spin locked up with its momentum at the crystal surface in the presence of large spin–orbit coupling. Resultantly, backscattering is notably suppressed which otherwise dominates in materials that are constantly associated with surface defects to some extent.16 Topological materials are therefore good candidates as catalysts because they possess high conductivity, topologically protected surface states, and suitable carrier concentration around the Fermi level.12,13 Furthermore, owing to their rich and exotic physical properties, they provide a model platform to explore the interplay among surface states, electron transfer, and surface catalytic reactions.12,17
It is now accepted that even an electron can act as a catalyst.18,19 Hence, altering the rate of a chemical reaction by manipulating the electrons on the catalyst surface would be an exciting way to control the kinetics of that reaction. Weyl semimetals are newly discovered quantum materials wherein the linearly dispersing bulk bands cross at Weyl points with finite chirality, with the total number of the positive chirality Weyl points being equal to that of the negative chirality.14 The discovery of Weyl semimetals has provided an ideal platform to test many previously unknown catalysts. The primary advantage of using Weyl semimetals as catalysts is their inherent property of very high charge mobility due to the linear crossing of bands at the Weyl points;14 besides, they contain special surface states that cannot be destroyed by breaking or scratching the surface because these states directly originate from the bulk electronic property.20 Furthermore, Weyl semimetals exhibit many other unique properties, including chiral anomaly and photogalvanic effect, owing to the inherent chirality of their electrons.12,21,22 Moreover, they show a strong response to an applied magnetic field, which is manifested as large magnetoresistance (MR) and magnetic-field-induced metal–insulator-like transition.23,24 In general, the abovementioned effects in Weyl semimetals have been observed at very low temperatures; however, in the case of the NbP family of Weyl semimetals, the considerably large MR effect and high electron mobility can be observed even at room temperature.21,23,25,26 Thus, studying the effect of an applied magnetic field on catalysis using this class of compounds is of great implication as we can observe the effect of change in electronic properties on the chemical reaction.
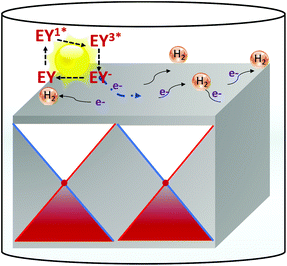
The hydrogen evolution reaction (HER) would be an ideal model catalytic reaction to study as it involves few intermediate steps and one product that is easy to monitor over the course of the reaction. Meanwhile, Weyl semimetals cannot perform self-sensitized hydrogen evolution. Therefore, Eosin Y assisted dye-sensitized photochemical hydrogen evolution was performed using NbP, TaP, NbAs, TaAs, and MoS2 as catalysts. In the presence of light, EY gets actuated to the excited singlet state EY1* followed by an inter-system crossing to the low-lying triplet state EY3*. Then, EY3* receives an electron from TEOA, transforming into a reducing species EY−, which then yields an electron to the catalyst surface where the HER takes place (Fig. 1).3,4 The role of the catalyst here is to accept the electron from the dye and transfer it to water for reduction. The rate of reaction is dependent on the electronic properties and the hydrogen binding energy of the catalyst. Since catalysts act as a channel for electrons from dye to water, the catalytic properties can be tuned by perturbing the electronic properties via an external field such as a magnetic field. Furthermore, we have performed a number of control experiments to conclude that the catalysts are hydrogen evolution sites and no other processes contribute to the HER (Table S1†).
Weyl semimetals can exhibit metallic conductivity along with considerably high electron mobility. Powdered forms of the single crystals of the compounds NbP, TaP, NbAs, and TaAs were characterized using X-ray powder diffraction and indexed (Fig. S1†). We first show the electronic properties of Weyl semimetal NbP and related compounds (TaP, NbAs, and TaAs) at room temperature. The transport properties of the single crystals of these compounds have recently been investigated in detail at cryogenic temperatures; however, in this study, we focus on properties relevant to our HER study at room temperature. The electron mobilities, resistivities, and MRs of the Weyl semimetals considered in our study at room temperature are listed in Table 1.
| Catalyst | Mobility (cm2 V−1 s−1) | Resistivity (10−5 Ω cm) | MR (%) |
|---|---|---|---|
| NbP | 275 | 6.30 | 253 |
| TaP | 849 | 2.53 | 279 |
| NbAs | 258 | 5.07 | 250 |
| TaAs | 385 | 4.75 | 129 |
All the compounds exhibit metallic conductivity, i.e., their resistivities decrease with decreasing temperature. As previously indicated, an important feature of these compounds is their large MR even at room temperature; MR is defined as the percentage increase in the resistivity of a material upon the application of a magnetic field. Fig. 2a shows the plot of MR as a function of an applied magnetic field. It is clear from the figure that the Weyl semimetal compounds considered in our study have a large MR value of 125–300% at room temperature, which is orders of magnitude higher than noble metals. Even at a low field of 0.5 T, the magnetoresistance is around 2–5% for these materials. Along with high mobility and large MR at room temperature, they also possess low hydrogen binding energy which would facilitate the rate of the reaction (Fig. 2b). Furthermore, photochemical hydrogen evolution with these materials previously studied show high HER activity due to metallic conductivity and high electron mobility.27 Hence, NbP, TaP, NbAs, and TaAs are ideal candidates for observing the effect of an applied magnetic field on their catalytic hydrogen evolution activity.
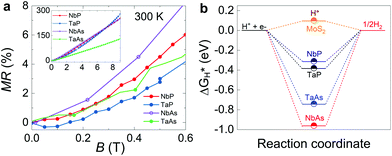 | ||
| Fig. 2 (a). Magnetoresistance (MR) of Weyl semimetals NbP, TaP, NbAs, and TaAs at room temperature and 0.6 T magnetic field. The inset shows the MR of Weyl semimetals at room temperature till 9 T. (b) Calculated free energy for the hydrogen evolution reaction relative to the standard hydrogen electrode at pH 0 for Weyl semimetals and MoS2. The values of Weyl semimetals and MoS2 are taken from ref. 27 and 28 respectively. | ||
Single crystals of the Weyl semimetals (NbP, TaP, NbAs, and TaAs) were powdered and characterized before conducting the hydrogen evolution studies. The particles are in the range of a few microns as inferred from FESEM images (Fig. S2†). The hydrogen evolution activity of these catalysts is recorded over the period and compared with MoS2, a diamagnetic semiconductor, and a well-known hydrogen evolution catalyst (the XRD and SEM images of MoS2 are shown in Fig. S1b and S4c†, respectively). In the case of MoS2, the total amount of hydrogen gas evolved in 3 h was 652 μmol g−1 and when a magnetic field is applied, there was a negligible change in the HER activity, with a yield of only 654 μmol g−1 in 3 h (Fig. 3a). We use the same reaction mixture in the cases with and without the magnet, and the only difference is the additional injection of the dye in the case with the magnet. Furthermore, to rule out the possibility that the additional dye added in the case of the second reaction is contributing to the HER activity enhancement using NbP, we reverse the order of our HER reactions, i.e., first, we perform the experiment with the magnet for 3 h, after which we remove the magnet and conduct the experiment again for 3 h. Upon removing the magnet, we observe an 88% decrease in the HER activity with NbP (Fig. 3b and c). When the field is applied later, the increment is 95% for the sample. Furthermore, to avoid any inconsistency, we performed all measurements for a particular sample on the same day at a constant temperature while keeping the location of the reaction vessel and lamp fixed throughout the experiment. The amount of hydrogen gas evolved for Weyl semimetals in 3 h is 333, 64, 88, and 81 μmol g−1 for NbP, TaP, NbAs, and TaAs without the magnetic field, respectively, whereas it increased to 652, 113, 98 and 110 μmol g−1 (Fig. S2a–d†) with the application of the magnetic field, which corresponds to an increase of 95, 77.4, 11.3, and 36.5%, respectively (Fig. 3d). We had checked the stability of the compounds after hydrogen evolution before and after exposure to the magnetic field for both MoS2 and NbP. We find no change in their morphology and chemical composition, indicating that the variation in the rate of the reaction is due to the manipulation of the electronic properties of the material by the magnet (Fig. S4†). Furthermore, the hydrogen evolution reaction is a complex process involving multiple steps and interplay between electronic surface states, electron transfer, and surface mass transfer reactions on the surface. The enhancement in the HER activity of the Weyl semimetals by the magnetic field shows that we can alter the rate of a chemical reaction by manipulating the participating electrons.
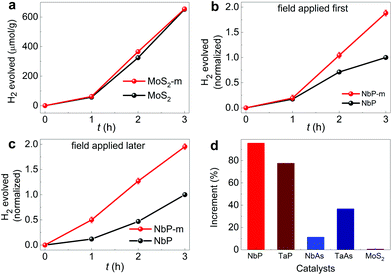 | ||
| Fig. 3 (a) Comparative hydrogen evolution for MoS2 over the period of time in the presence of the magnetic field (m) and absence. Normalized hydrogen evolution of NbP with (m) and without magnetic field for cases (b) magnetic field applied first, (c) magnetic field applied later. (d) The comparison of the increment in the magnetic field for Weyl semimetals and MoS2. The hydrogen evolution graphs over the period are shown in Fig. S3.† The applied magnetic field is 3500 G. The same part of the crystal was used for the studies. | ||
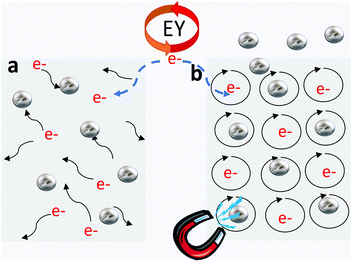
Based on our observations, it is clear that a magnetic field affects the HER activity when diamagnetic Weyl semimetals are used as catalysts; this effect is of interest considering that nonmagnetic materials were used for these experiments. Nevertheless, our results are consistent with the idea that the electronic properties of Weyl semimetals can be manipulated using a magnetic field. In this regard, nonmagnetic Weyl semimetals have earlier been shown to exhibit a negative MR induced by electron transport between two Weyl nodes of opposite chirality even at room temperature.29 Electrons in metals, including topological Weyl semimetals, get localized upon the application of a magnetic field, which can also be understood in terms of the circular motion of electrons in a plane perpendicular to the direction of the applied magnetic field (Fig. 4).
The high mobility of electrons in Weyl semimetals ensures that such motion can persist for a considerable time before they are scattered (i.e., long scattering time). These states are expected to be readily available for catalytic processes, leading to an increase in the HER activity under a magnetic field. However, further understanding of the mechanism of catalytic activity under a magnetic field and the manner in which it is related to the Berry curvature of topological Weyl semimetals are important topics for future research.
Conclusions
We observed a large enhancement in the hydrogen evolution catalytic activity of nonmagnetic Weyl semimetals of the NbP family upon the application of a magnetic field. The magnetic field almost doubled the HER activity of NbP; in addition, significant increases in the HER activity were also noted for other catalysts of this family. Such large variations in the catalytic activity of these semimetals indicate a drastic evolution of the bulk and surface electronic properties upon the application of a magnetic field, which is also reflected as a large MR effect at room temperature. The slight application of a magnetic field can alter the rate of the reaction significantly. It would be interesting to study the effect on catalysis by varying the magnetic field and understanding the exact mechanism via in situ experiments. Manipulation of electronic properties of catalysts may serve as an alternative route to change the rate of the reaction without affecting the reaction pathway which can be further explored for other chemical reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the ERC Advanced Grant No. 742068 ‘TOPMAT’. Dr U. Burkhardt for the SEM measurements. Open Access funding provided by the Max Planck Society.References
- T. Banerjee, F. Haase, G. Savasci, K. Gottschling, C. Ochsenfeld and B. V. Lotsch, J. Am. Chem. Soc., 2017, 139, 16228–16234 CrossRef CAS PubMed.
- Y. Jing and T. Heine, J. Mater. Chem. A, 2018, 6, 23495–23501 RSC.
- U. Maitra, U. Gupta, M. De, R. Datta, A. Govindaraj and C. N. R. Rao, Angew. Chem., Int. Ed., 2013, 52, 13057–13061 CrossRef CAS PubMed.
- U. Gupta, B. S. Naidu, U. Maitra, A. Singh, S. N. Shirodkar, U. V. Waghmare and C. N. R. Rao, APL Mater., 2014, 2, 092802 CrossRef.
- D. Voiry, M. Salehi, R. Silva, T. Fujita, M. Chen, T. Asefa, V. B. Shenoy, G. Eda and M. Chhowalla, Nano Lett., 2013, 13, 6222–6227 CrossRef CAS.
- X. Ren, J. Zhou, X. Qi, Y. Liu, Z. Huang, Z. Li, Y. Ge, S. C. Dhanabalan, J. S. Ponraj, S. Wang, J. Zhong and H. Zhang, Adv. Energy Mater., 2017, 7, 1700396 CrossRef.
- Q. Jiang, L. Xu, N. Chen, H. Zhang, L. Dai and S. Wang, Angew. Chem., 2016, 128, 14053 CrossRef.
- W. Han, C. Zang, Z. Huang, H. Zhang, L. Ren, X. Qi and J. Zhong, Int. J. Hydrogen Energy, 2014, 39, 19502–19512 CrossRef CAS.
- Z. Zhang, Y. Liu, L. Ren, H. Zhang, Z. Huang, X. Qi, X. Wei and J. Zhong, Electrochim. Acta, 2016, 200, 142–151 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- C. Huang, C. Li and G. Shi, Energy Environ. Sci., 2012, 5, 8848–8868 RSC.
- G. Li, Q. Xu, W. Shi, C. Fu, L. Jiao, M. E. Kamminga, M. Yu, H. Tüysüz, N. Kumar, V. Süβ, R. Saha, A. K. Srivastava, S. Wirth, G. Auffermann, J. Gooth, S. Parkin, Y. Sun, E. Liu and C. Felser, Sci. Adv., 2019, 5, eaaw9867 CrossRef PubMed.
- G. Li, C. Fu, W. Shi, L. Jiao, J. Wu, Q. Yang, R. Saha, M. E. Kamminga, A. K. Srivastava, E. Liu, A. N. Yazdani, N. Kumar, J. Zhang, G. R. Blake, X. Liu, M. Fahlman, S. Wirth, G. Auffermann, J. Gooth, S. Parkin, V. Madhavan, X. Feng, Y. Sun and C. Felser, Angew. Chem., Int. Ed., 2019, 58, 13107–13112 CrossRef CAS PubMed.
- B. Yan and C. Felser, Annu. Rev. Condens. Matter Phys., 2017, 8, 337–354 CrossRef.
- L. Müchler, H. Zhang, S. Chadov, B. Yan, F. Casper, J. Kübler, S.-C. Zhang and C. Felser, Angew. Chem., Int. Ed., 2012, 51, 7221–7225 CrossRef PubMed.
- L. M. Schoop, F. Pielnhofer and B. V. Lotsch, Chem. Mater., 2018, 30, 3155–3176 CrossRef CAS.
- M. Z. Hasan, S.-Y. Xu, I. Belopolski and S.-M. Huang, Annu. Rev. Condens. Matter Phys., 2017, 8, 289–309 CrossRef CAS.
- A. Studer and D. P. Curran, Nat. Chem., 2014, 6, 765 CrossRef CAS PubMed.
- K. N. Houk and B. List, Acc. Chem. Res., 2004, 37, 487–487 CrossRef CAS.
- X. Wan, A. M. Turner, A. Vishwanath and S. Y. Savrasov, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 205101 CrossRef.
- X. Huang, L. Zhao, Y. Long, P. Wang, D. Chen, Z. Yang, H. Liang, M. Xue, H. Weng, Z. Fang, X. Dai and G. Chen, Phys. Rev. X, 2015, 5, 031023 Search PubMed.
- Q. Ma, S.-Y. Xu, C.-K. Chan, C.-L. Zhang, G. Chang, Y. Lin, W. Xie, T. Palacios, H. Lin, S. Jia, P. A. Lee, P. Jarillo-Herrero and N. Gedik, Nat. Phys., 2017, 13, 842 Search PubMed.
- C. Shekhar, A. K. Nayak, Y. Sun, M. Schmidt, M. Nicklas, I. Leermakers, U. Zeitler, Y. Skourski, J. Wosnitza, Z. Liu, Y. Chen, W. Schnelle, H. Borrmann, Y. Grin, C. Felser and B. Yan, Nat. Phys., 2015, 11, 645 Search PubMed.
- M. N. Ali, J. Xiong, S. Flynn, J. Tao, Q. D. Gibson, L. M. Schoop, T. Liang, N. Haldolaarachchige, M. Hirschberger, N. P. Ong and R. Cava, Nature, 2014, 514, 205 CrossRef CAS PubMed.
- N. J. Ghimire, Y. Luo, M. Neupane, D. J. Williams, E. D. Bauer and F. Ronning, J. Phys.: Condens. Matter, 2015, 27, 152201 CrossRef CAS PubMed.
- F. Arnold, C. Shekhar, S.-C. Wu, Y. Sun, R. D. dos Reis, N. Kumar, M. Naumann, M. O. Ajeesh, M. Schmidt, A. G. Grushin, J. H. Bardarson, M. Baenitz, D. Sokolov, H. Borrmann, M. Nicklas, C. Felser, E. Hassinger and B. Yan, Nat. Commun., 2016, 7, 11615 CrossRef CAS PubMed.
- C. R. Rajamathi, U. Gupta, N. Kumar, H. Yang, Y. Sun, V. Süβ, C. Shekhar, M. Schmidt, H. Blumtritt, P. Werner, B. Yan, S. Parkin, C. Felser and C. N. R. Rao, Adv. Mater., 2017, 29, 1606202 CrossRef PubMed.
- B. Hinnemann, P. G. Moses, J. Bonde, K. P. Jørgensen, J. H. Nielsen, S. Horch, I. Chorkendorff and J. K. Nørskov, J. Am. Chem. Soc., 2005, 127, 5308–5309 CrossRef CAS.
- A. C. Niemann, J. Gooth, S.-C. Wu, S. Bäßler, P. Sergelius, R. Hühne, B. Rellinghaus, C. Shekhar, V. Süβ, M. Schmidt, C. Felser, B. Yan and K. Nielsch, Sci. Rep., 2017, 7, 43394 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/D0DT00050G |
| This journal is © The Royal Society of Chemistry 2020 |