Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Increased photocurrent of CuWO4 photoanodes by modification with the oxide carbodiimide Sn2O(NCN)†
Zheng
Chen
a,
Manuel
Löber
b,
Anna
Rokicińska
 c,
Zili
Ma
c,
Zili
Ma
 a,
Jianhong
Chen
a,
Jianhong
Chen
 d,
Piotr
Kuśtrowski
d,
Piotr
Kuśtrowski
 c,
Hans-Jürgen
Meyer
c,
Hans-Jürgen
Meyer
 b,
Richard
Dronskowski
b,
Richard
Dronskowski
 ae and
Adam
Slabon
ae and
Adam
Slabon
 *d
*d
aChair of Solid-State and Quantum Chemistry, Institute of Inorganic Chemistry, RWTH Aachen University, 52056 Aachen, Germany
bSection of Solid State and Theoretical Inorganic Chemistry, Institute of Inorganic Chemistry, University of Tübingen, 72076 Tübingen, Germany
cFaculty of Chemistry, Jagiellonian University, 30-387 Krakow, Poland
dDepartment of Materials and Environmental Chemistry, Stockholm University, 10691 Stockholm, Sweden. E-mail: adam.slabon@mmk.su.se
eHoffmann Institute of Advanced Materials, Shenzhen Polytechnic, 7098 Liuxian Blvd, Shenzhen, China
First published on 18th February 2020
Abstract
Tin(II) oxide carbodiimide is a novel prospective semiconductor material with a band gap of 2.1 eV and lies chemically between metal oxides and metal carbodiimides. We report on the photochemical properties of this oxide carbodiimide and apply the material to form a heterojunction with CuWO4 thin films for photoelectrochemical (PEC) water oxidation. Mott–Schottky experiments reveal that the title compound is an n-type semiconductor with a flat-band potential of −0.03 V and, as such, the position of the valence band edge would be suitable for photochemical water oxidation. Sn2O(NCN) increases the photocurrent of CuWO4 thin films from 32 μA cm−2 to 59 μA cm−2 at 1.23 V vs. reversible hydrogen electrode (RHE) in 0.1 M phosphate buffer (pH 7.0) under backlight AM 1.5G illumination. This upsurge in photocurrent originates in a synergistic effect between the oxide and oxide carbodiimide, because the heterojunction photoanode displays a higher current density than the sum of its individual components. Structural analysis by powder X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) reveals that Sn2O(NCN) forms a core–shell structure Sn2O(NCN)@SnPOx during the PEC water oxidation in phosphate buffer. The electrochemical activation is similar to the behavior of Mn(NCN) but different from Co(NCN).
Introduction
One of the critical technical problems facing humanity is the development of a long-term sustainable energy economy.1 This especially includes clean and renewable energy generation; a task that can be theoretically fulfilled by photoelectrochemical (PEC) water-splitting to yield hydrogen.2 Water-splitting consists of two half reactions: the transfer of two electrons for the hydrogen evolution reaction (HER) and four electrons for the oxygen evolution reaction (OER).3 The photo-generated charge and holes separate and migrate through the semiconductor in opposite directions to generate a photocurrent. Since the initial report on solar-driven water-splitting on a TiO2 photoelectrode in 1972,4 metal oxide semiconductors have been intensively investigated, and BiVO4 has emerged as the benchmark oxide photoanode.5 There are only few known oxidic photoanode candidates that display a smaller band gap than BiVO4, such as CuWO4 with an electronic band gap in the range of 2.2–2.4 eV.6There are several critical characteristics for a semiconductor material in terms of realistic application as a water-splitting photoelectrode: (i) suitable conduction and/or valence band edge positions; (ii) stability under operating conditions; (iii) efficient charge carrier separation and transport along and across the thin-film electrode; (iv) low-cost and earth-abundant elements; and (v) sufficient light absorption.5–7 Additionally, the surface of the light absorber has to be modified in most cases with a suitable catalyst to overcome poor reaction kinetics, i.e. to drive the HER and/or OER.8
Copper tungstate is one of the few promising photoanode materials with a suitable band gap, a valence band edge (VBE) more positive than 1.23 V vs. RHE to permit water oxidation, stability under neutral conditions and composed of abundant elements.9 It can be manufactured on differential electrically conductive substrates by electrochemical deposition,10 atomic layer deposition (ALD),11 spray pyrolysis,12 and spin-casting.13 Several strategies have been reported to improve the performance of CuWO4 by doping with zinc,14 molybdenum15 or iron,12 incorporating with silver nanowires,16 functionalizing with gold nanoparticles.17 Charge transport is also facilitated by post-synthetic hydrogen or nitrogen treatment of CuWO4 due to the formation of oxygen vacancies.10,18 Recently, we have reported that surface modification of CuWO4 with Ag2(NCN)8a or Mn(NCN)8b displays synergetic effects between its constituents, which improve PEC water oxidation efficiency.
Carbodiimides have received attention as novel materials for photochemical energy conversion in addition to their application as electrode materials for Li-ion batteries.19 This has been mainly motivated by their suitable band gap values for solar light harvesting and a beneficial VBE position for PEC water oxidation.20 They are related to oxides but are characterized by a higher degree of covalency.21 The carbodiimide anion (−N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N−) is considered as a pseudo-chalcogenide anion and lies between oxide and sulfide anions in view of the HSAB concept.22 The oxide carbodiimides are as such mixed-anion compounds. The oxide carbodiimide representative Sn2O(NCN) was obtained recently by Meyer et al. as crystalline powder with an optical band gap of approximately 2.0 eV.23 The title compound is closely related to tin(II) oxide (SnO); a semiconductor with a band gap of 2.8 eV as calculated on the basis of electronic band structure calculations.24
N−) is considered as a pseudo-chalcogenide anion and lies between oxide and sulfide anions in view of the HSAB concept.22 The oxide carbodiimides are as such mixed-anion compounds. The oxide carbodiimide representative Sn2O(NCN) was obtained recently by Meyer et al. as crystalline powder with an optical band gap of approximately 2.0 eV.23 The title compound is closely related to tin(II) oxide (SnO); a semiconductor with a band gap of 2.8 eV as calculated on the basis of electronic band structure calculations.24
In this work, we report on the photochemical properties of Sn2O(NCN) and the fabrication of heterojunction CuWO4/Sn2O(NCN) thin film photoanodes. We show that Sn2O(NCN) undergoes structural changes during PEC water oxidation and can augment the photocurrent of CuWO4 photoanodes.
Experimental
Synthesis of Sn2O(NCN)
Li2(NCN) was prepared as previously reported by Meyer.23,25 Equimolar amounts of Li2(NCN), Na2O and SnCl2 (Sigma Aldrich 99.999%, ultra dry) were mixed and ground in an agate mortar under argon. Samples of 250 mg were sealed into silica tubes under vacuum and each mixture was heated in a furnace to temperatures ranging from 450 to 500 °C. The samples were kept for 12 h before cooling to room temperature. The ampoules were opened in air, the product was washed with deionized water and dried in oven at 80 °C for 4 h. Sn2O(NCN) was obtained as a red powder.Synthesis of CuWO4 thin films
CuWO4 thin films were produced on conductive fluorine-doped tin oxide (FTO) glass (2.0 mm thick, Sigma-Aldrich), based on a synthesis by Bartlett.9a Before the electrochemical synthesis, the FTO glass was cleaned in diluted nitric acid (Sigma), acetone and ethanol, respectively. 1.26 g (3.8 mmol) sodium tungstate dihydrate (Na2WO4·2H2O, 99.9%, Acros Organics) was dissolved in 15 mL deionized water by stirring, and 1 mL hydrogen peroxide (30%, Geyer Chemsolute) was added to the tungstate precursor solution. The solution was stirred for 20 min at room temperature. 25 mL deionized water and 25 mL isopropanol (>99.7%, Fisher Scientific) were added to the solution. A solution of 0.73 g (2.7 mmol) copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, >99%, Sigma) in 10 mL deionized water was added to the tungsten precursor solution. The pH value was adjusted to 1.2 by adding nitric acid and the solution was used for electrochemical deposition on FTO glass. The electrochemical deposition was performed in a three-electrode setup with platinum wire and 1 M Ag/AgCl (WAT Venture) as a counter electrode and a reference electrode, respectively. The electrochemical deposition was carried out by a Gamry potentiostat and the Gamry framework software package. The potential was swept in the range from −0.9 to +0.2 V vs. 1 M Ag/AgCl for 12 cycles at the scan rate of 50 mV s−1. The working electrode was disconnected in the electrical circuit, washed with deionized water and dried at room temperature under vacuum. The working electrode was heated at 450 °C for 2 h under ambient atmosphere. The excess of copper oxides was etched by immersing the working electrode into 0.5 M HCl for acidic treatment. The electrode was subsequently annealed one more time at 450 °C for 30 min under ambient atmosphere.Preparation of Sn2O(NCN) and CuWO4/Sn2O(NCN) photoanodes
Sn2O(NCN) powder was dispersed in ethanol (120 μg mL−1) by ultrasounds. FTO, a bare graphite (for XPS) and CuWO4 thin film electrode were placed on a heating plate at 50 °C. The Sn2O(NCN) dispersion was drop-casted on the surfaces of the corresponding thin film electrodes.Structural characterization
Powder XRD patterns were recorded in transmission mode on a STOE STADI-P diffractometer (Cu Kα1 radiation) operating with a DECTRIS Mythen 1K detector. For the analysis of the photoanodes by XRD, the samples were mechanically removed from the photoanodes in advance. SEM images of Sn2O(NCN) powder were recorded by a Leo Supra 35VP SMT (Zeiss).A Themis Z TEM (Thermo Fisher) equipped with a SuperX energy dispersive X-ray (EDX) detector operated at 300 kV in the scanning TEM mode was used for determination of the chemical composition of Sn2O(NCN) particles, which were subject to chronoamperometry at 1.23 V vs. RHE. Prior to the analysis, the particles were mechanically removed from the pure Sn2O(NCN) electrode.
XPS spectra were collected by a hemispherical VG SCIENTA R3000 analyzer using a monochromatized aluminum source Al Kα (E = 1486.6 eV) at constant pass energy of 100 eV. The binding energies were referenced to the Au 4f core level (Eb = 84.0 eV). The composition and chemical surrounding of the sample surface were determined on the basis of the areas and binding energies of Na 1s, K 2p, P 2p, O 1s, N 1s, C 1s and Sn 3d photoelectron peaks. The fitting of high resolution spectra was obtained by using the Casa XPS software. UV-Vis spectra were recorded on a Shimadzu UV-2600 spectrophotometer. Measurements were recorded in absorbance and reflectance mode. The Tauc plots were calculated by Kubelka–Munk function F(R) = (1 − R)2/2R to determine the electronic band gap.
Photoelectrochemistry
The experiments were carried out in an electrochemical cell operating in a three-electrode setup. The photoanode was used as a working electrode. Platinum wire and a 1 M Ag/AgCl electrode were used as a counter electrode and a reference electrode, respectively. All current values of the electrodes were recorded vs. 1 M Ag/AgCl reference electrode and converted vs. RHE according to ERHE (V) = E1 M Ag/AgCl (V) + 0.236 (V) + [0.059 × pH] (V) at 25 °C. The electrochemical data were recorded by a potentiostat (Gamry instruments). A solar light simulator (class-AAA 94023A, Newport) with an ozone-free 450 W xenon short-arc lamp was used to illuminate the photoanode with 100 mW cm−2 (AM 1.5G) simulated visible light. The power output of the solar simulator was calibrated with a Si reference cell (LOT-Quantum Design, Germany). 0.1 M potassium/sodium phosphate (K/NaPi) buffer (or with 0.05 M Na2SO3 as hole scavenger) at pH 7.0 was used as the electrolyte for PEC experiments and prepared with Milli-Q water (18.3 Ω cm) at 25 °C. The linear square voltammetry (LSV) and cyclic voltammetry (CV) were swept at a scan rate of 10 mV s−1 from 0.64 to 1.44 V vs. RHE. Mott–Schottky (MS) measurements were carried out under dark in an electromagnetically shielded box. A sinusoidal modulation of 10 mV was applied at frequencies of 10 Hz, 100 Hz and 1000 Hz in the potential range from 0.05 V to 1.23 V vs. RHE with an equilibration time of 10 s.Results and discussion
Structure characterization of the bulk
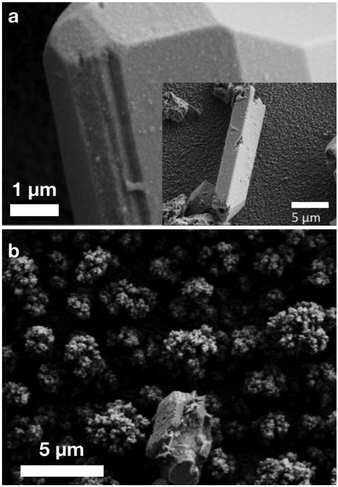
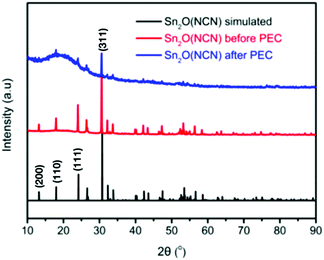
Fig. 1a shows SEM images of the obtained microcrystalline Sn2O(NCN) and the thin film CuWO4/Sn2O(NCN) photoanode. Sn2O(NCN) crystallizes in an orthorhombic crystal structure (space group Pccn) and has Sn2+ ions situated in a fourfold, mixed O2−/NCN2− coordination environment.23 The carbodiimide anion is closely related to the oxide anion and can be regarded as an N-based pseudo-oxide.26 For structural characterization, we tested the photoanodes for PEC water oxidation (vide infra) and removed subsequently a part of the thin film for XRD and XPS characterization. The powder XRD patterns of a pure Sn2O(NCN) photoanode indicate that the compound maintains its structural bulk stability after PEC water oxidation in phosphate electrolyte at pH 7.0 (Fig. 2).The corresponding powder XRD patterns of the composite CuWO4/Sn2O(NCN) photoanodes do not exhibit reflection peaks of the oxide carbodiimide due to the low amount of the latter (Fig. 3). The XRD patterns before and after PEC operation remain unchanged and match the simulated patterns of the copper tungstate.
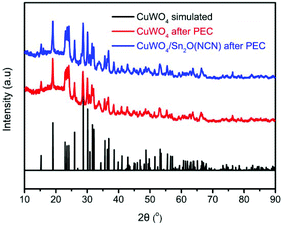 | ||
| Fig. 3 Experimental and simulated powder XRD patterns of CuWO4 and CuWO4/Sn2O(NCN) after PEC water oxidation. | ||
Electronic structure
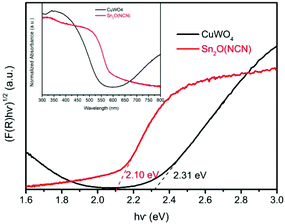
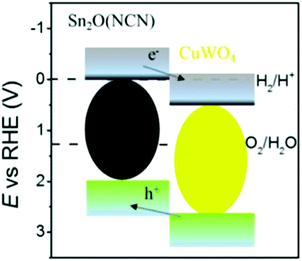
The electronic band gaps of CuWO4 and Sn2O(NCN) were determined by the Kubelka–Munk function. The results show that the band gap of CuWO4 and Sn2O(NCN) are 2.31 and 2.10 eV, respectively (Fig. 4). Both determined values are close to the previously reported values of 2.2–2.4 eV for CuWO414 and 2.0 eV for Sn2O(NCN).23 This would render both compounds as potential photoanode candidates for water oxidation.The semiconducting type and band edge positions of Sn2O(NCN) were determined by MS measurements. Fig. 5 illustrates the MS plots of Sn2O(NCN) photoanodes for applied different frequencies of 10, 100 and 1000 Hz. All curves exhibit a positive slope, indicating that the oxide carbodiimide Sn2O(NCN) is an n-type semiconductor; similar to CuWO4. For all three different frequencies, extrapolation of the measured data yields a flat-band potential of −0.03 V vs. RHE. For an n-type semiconductor, the value of the flat-band potential is close to the conduction band edge (CBE) position. Taking into account the determined band gap, the VBE and CBE positions are 2.07 V and −0.03 V vs. RHE, respectively. A comparison of the CBE and VBE positions for the binary tin oxides is shown in Fig. S1.† Similar to SnO2, the oxide carbodiimide would be theoretically suited for overall water-splitting.24,27
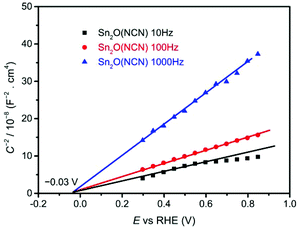 | ||
| Fig. 5 MS analysis of EIS measurements of Sn2O(NCN) electrodes applied frequencies of 10, 100 and 1000 Hz. | ||
Photoelectrochemistry
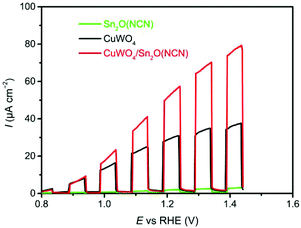
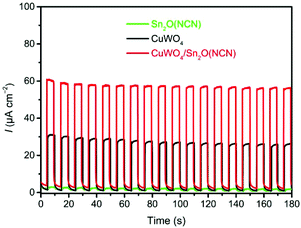
For the investigation of heterojunction photoanodes CuWO4/Sn2O(NCN), two additional electrodes of the individual components were produced. All measurements were performed in phosphate electrolyte at pH 7.0. The summarized LSV curves for all three electrodes, recorded with a scan rate at 10 mV s−1 under backlight AM 1.5G illumination (100 mW cm−2), are depicted in Fig. 6. The CuWO4 photoanode developed an anodic current that reached 32 μA cm−2 at 1.23 V vs. RHE.Upon functionalizing the surface with Sn2O(NCN) particles by drop-casting, the photocurrent showed an upsurge which was reached when adding 36 μg (Fig. S2†). A bare Sn2O(NCN) electrode with the same amount of material as for the composite photoanode CuWO4/Sn2O(NCN) developed only a small photocurrent. The photocurrent produced by the heterojunction electrode, being equal 59 μA cm−2 at 1.23 V vs. RHE, exceeds the sum of its individual components and exhibits as such a synergistic effect. This trend is more visible during chronoamperometry (CA) with interrupted illumination (Fig. 7). The photocurrent remains relatively stable for a tested period of 2 hours (Fig. S3†).
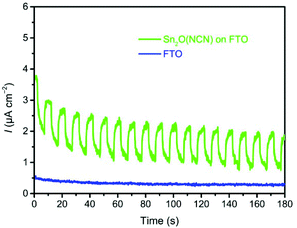
Since the photocurrent of the oxide carbodiimide was very small, we created another Sn2O(NCN) photoanode by electrophoretic deposition with a higher amount of material. Fig. 8 illustrates the results of CA for this Sn2O(NCN) photoanode and FTO glass, as a reference measurement, at 1.23 V vs. RHE in the same electrolyte under illumination. It can be clearly seen that the photocurrent of Sn2O(NCN) outperforms the substrate. As such one can rule out that the photoactivity of Sn2O(NCN) may be due to surface oxidation toward a tin oxide phase.
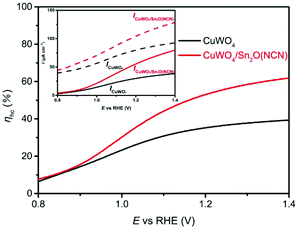
In order to elucidate the origin of increased photocurrents after Sn2O(NCN) functionalization, we determined the hole collection efficiency ηhc. The oxidation of a hole scavenger, such as the sulfite anion, allows to determine the number of surface-reaching holes because this oxidation reaction is much faster than the sluggish oxidation of water. This allows to estimate that each hole reaching the semiconductor–electrolyte interface will be used for an oxidative reaction. The comparison of the photocurrents for sulfite oxidation (JNa2SO3) and water oxidation (JH2O) allows to calculate ηhc = (JH2O/JNa2SO3). For the CuWO4 thin films, the ηhc value significantly increases upon functionalization with Sn2O(NCN) at higher potentials (Fig. 9). This indicates that the reactivity of the surface increases upon functionalization with the oxide carbodiimide.
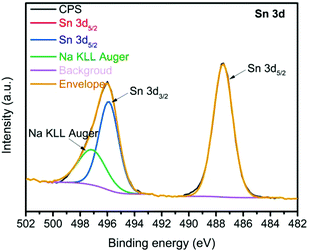
To investigate the key factors of improved PEC performance, we analyzed the surface of bare Sn2O(NCN) photoanodes after the PEC experiment by XPS. The electrode was prepared on graphite instead of FTO substrate to avoid interference with Sn stemming from FTO. Except the presence of O, C, N and Sn, XPS reveals the presence of Na, K and P which originate from the K/NaPi electrolyte. In Fig. 10, the high-resolution XPS Sn 3d spectrum, with the Sn 3d5/2 and Sn 3d3/2 peaks distanced at the splitting energy of 8.4 eV, is shown. The latter one is partially overlapped with the Na KLL Auger signal. The relative high values of binding energies found for both components (487.5 eV for Sn 3d5/2 and 495.9 eV for Sn 3d3/2) obviously suggest that Sn2O(NCN) formed a phosphate-type shell on the surface being exposed to the phosphate electrolyte, i.e. a core–shell structure Sn2O(NCN)@SnPOx. This indicates that the catalytically active form is a tin phosphate shell while the core contains the semiconducting Sn2O(NCN). Similar positions of the Sn 3d signals have been observed recently for tin phosphate (SnPOx) in relation to SnO2, which exhibited the Sn 3d5/2 and Sn 3d3/2 components at 486.0 eV and 494.4 eV, respectively.28 Different from the behavior of Co(NCN) electrocatalysts,29 the electrochemical activation is similar to the behavior of Mn(NCN) electrocatalysts8b as previously reported. Tin phosphate is known as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid according to the literature report. The fourfold coordinated Sn4+ sites from tin phosphate are identified as the active species.28
The presence of phosphorous was also confirmed by complementary TEM EDX analysis, which was performed on Sn2O(NCN) particles that were mechanically removed from a FTO/Sn2O(NCN) thin film electrode after 30 min of CA at 1.23 V vs. RHE. The high-angle annular dark field (HAADF) images in Fig. 11 shows agglomerated particles that were scanned by means of EDX. Besides phosphorous, the presence of tin, oxygen, carbon and nitrogen could be confirmed, too.
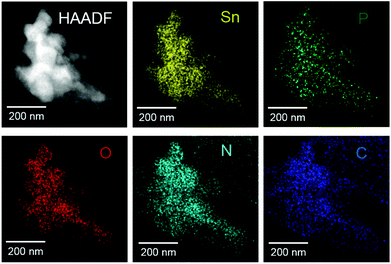 | ||
| Fig. 11 HAADF image and corresponding elemental maps of Sn2O(NCN) particles that were subject to CA at 1.23 V vs. RHE. | ||
The augmented charge carrier separation for the heterojunction can be understood by analyzing the energy band diagram of both semiconductors (Fig. 12).8a The energetically higher position of the CBE for Sn2O(NCN) in comparison to CuWO4 enables injection of electrons into the conduction band of the latter. At the same time, the photogenerated holes can diffuse from the VBE of CuWO4 to Sn2O(NCN). This results in decreased recombination of photogenerated electrons and holes. In addition to the improved hole collection efficiency, being the consequence of the tin phosphate shell, the PEC water oxidation efficiency could be further increased by 10% when depositing cobalt phosphate as co-catalyst on the surface of the heterojunction photoanode (Fig. S4†).
Conclusions
We have investigated the photochemical properties of Sn2O(NCN) and showed that this n-type semiconductor can be successfully coupled to CuWO4 thin film photoanodes. Sn2O(NCN) exhibits a flat-band potential of approx. −0.03 V and as such, the position of the valence band edge would be suitable for photochemical water oxidation. During PEC water oxidation in phosphate buffer electrolyte Sn2O(NCN) undergoes an in situ transformation to a core–shell structure; maintaining a semiconducting core while forming an electrocatalytically-active SnPOx shell. The obtained composite CuWO4/Sn2O(NCN)@SnPOx photoanodes display a synergetic effect between its constituents during the PEC water oxidation, which shows up in an upsurge of photocurrent from 32 μA cm−2 to 59 μA cm−2 at 1.23 V vs. RHE at pH 7.0 under simulated AM 1.5G illumination. This is due to improved charge carrier separation and augmented hole collection efficiency. Our study demonstrates that mixed-anion compounds containing an oxidic and carbodiimide anion are potential materials for photochemical oxidation reactions, while at the same time, the surface can be electrochemically activated into a catalytically-active form.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Zh. C. would like to thank the China Scholarship Council for a PhD scholarship. We thank Ulrich Simon for access to electron microscopy facilities, Birgit Hahn for SEM analysis, and Marek Drozdek for performing XPS, and Istvan-Zoltan Jenei for fruitful discussion. A. S. thank the Fonds der Chemischen Industrie (FCI) and Stockholm University for financial support. The XPS measurements were carried out with the equipment purchased with the financial support of the European Regional Development Fund in the framework of the Polish Innovation Operational Program (contract no. POIG.02.01.00-12-023/08).References
- J. P. Holdren, Science, 2007, 315, 737 CrossRef CAS PubMed.
- (a) N. S. Lewis, Nat. Nanotechnol., 2016, 11, 1010–1019 CrossRef CAS PubMed; (b) M. Grätzel, Nature, 2001, 414, 338–344 CrossRef PubMed; (c) Z. Ma, T. Thersleff, A. L. Görne, N. Cordes, Y. Liu, S. Jakobi, A. Rokicinska, Z. G. Schichtl, R. H. Coridan, P. Kuśtrowski, W. Schnick, R. Dronskowski and A. Slabon, ACS Appl. Mater. Interfaces, 2019, 11, 19077–19086 CrossRef CAS PubMed.
- K. Sivula and R. van de Krol, Nat. Rev. Mater., 2016, 1, 15010 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- (a) Z. Wang, C. Li and K. Domen, Chem. Soc. Rev., 2019, 48, 2109–2125 RSC; (b) C. Jiang, S. J. A. Moniz, A. Wang, T. Zhang and J. Tang, Chem. Soc. Rev., 2017, 46, 4645–4660 RSC; (c) H. S. Han, S. Shin, D. H. Kim, I. J. Park, J. S. Kim, P. S. Huang, J. K. Lee, I. S. Cho and X. Zheng, Energy Environ. Sci., 2018, 11, 1299–1306 RSC; (d) T. R. Kuo, H. J. Liao, Y. T. Chen, C. Y. Wei, C. C. Chang, Y. C. Chen, Y. H. Chang, J. C. Lin, Y. C. Lee, C. Y. Wenand and S. S. Li, Green Chem., 2018, 20, 1640–1647 RSC.
- (a) D. K. Lee, D. Lee, M. A. Lumley and K. S. Choi, Chem. Soc. Rev., 2019, 48, 2126–2157 RSC; (b) J. H. Kim and J. S. Lee, Adv. Mater., 2019, 31, 1806938 CrossRef PubMed.
- (a) K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef CAS PubMed; (b) N. S. Lewis, Science, 2007, 315, 798 CrossRef CAS PubMed; (c) M. Ertl, Z. Ma, T. Thersleff, P. Lyu, S. Huettner, P. Nachtigall, J. Breu and A. Slabon, Inorg. Chem., 2019, 58, 9655–9662 CrossRef CAS PubMed.
- (a) M. Davi, A. Drichel, M. Mann, T. Scholz, F. Schrader, A. Rokicinska, P. Kuśtrowski, R. Dronskowski and A. Slabon, J. Phys. Chem. C, 2017, 121, 26265–26274 CrossRef; (b) M. Davi, M. Mann, Z. Ma, F. Schrader, A. Drichel, S. Budnyk, A. Rokicinska, P. Kuśtrowski, R. Dronskowski and A. Slabon, Langmuir, 2018, 34, 3845–3852 CrossRef CAS PubMed; (c) M. Davi, F. Schrader, T. Scholz, Z. Ma, A. Rokicinska, R. Dronskowski, P. Kuśtrowski and A. Slabon, ACS Appl. Nano Mater., 2018, 1, 869–876 CrossRef CAS; (d) M. Davi, G. Ogutu, F. Schrader, A. Rokicinska, P. Kuśtrowski and A. Slabon, Eur. J. Inorg. Chem., 2017, 37, 4267–4274 CrossRef.
- (a) J. E. Yourey and B. M. Bartlett, J. Mater. Chem., 2011, 21, 7651–7660 RSC; (b) V. Leute, Z. Phys. Chem., 1966, 48, 319–339 CrossRef CAS.
- Z. Ma, O. Linnenberg, A. Rokicinska, P. Kuśtrowski and A. Slabon, J. Phys. Chem. C, 2018, 122, 19281–19288 CrossRef CAS.
- (a) X. Zhou, R. Liu, K. Sun, K. M. Papadantonakis, B. S. Brunschwig and N. S. Lewis, Energy Environ. Sci., 2016, 9, 892–897 RSC; (b) Y. Gao, O. Zandi and T. W. Hamann, J. Mater. Chem. A, 2016, 4, 2826–2830 RSC; (c) X. Zhou, R. Liu, K. Sun, D. Friedrich, M. T. McDowell, F. Yang, S. T. Omelchenko, F. H. Saadi, A. C. Nielander, S. Yalamanchili, K. M. Papadantonakis, B. S. Brunschwig and N. S. Lewis, Energy Environ. Sci., 2015, 8, 2644–2649 RSC.
- D. Bohra and W. A. Smith, Phys. Chem. Chem. Phys., 2015, 17, 9857–9866 RSC.
- (a) J. E. Yourey, K. J. Pyper, J. B. Kurtz and B. M. Bartlett, J. Phys. Chem. C, 2013, 117, 8708–8718 CrossRef CAS; (b) C. R. Lhermitte and B. M. Bartlett, Acc. Chem. Res., 2016, 49, 1121–1129 CrossRef CAS PubMed.
- J. E. Yourey, J. B. Kurtz and B. M. Bartlett, Inorg. Chem., 2012, 51, 10394–10401 CrossRef CAS PubMed.
- J. C. Hill, Y. Ping, G. A. Galli and K. S. Choi, Energy Environ. Sci., 2013, 6, 2440–2446 RSC.
- H. Zhang, P. Yilmaz, J. O. Ansari, F. F. Khan, R. Binions, S. Krause and S. Dunn, J. Mater. Chem. A, 2015, 3, 9638–9644 RSC.
- M. Valenti, D. Dolat, G. Biskos, A. Schmidt-Ott and W. A. Smith, J. Phys. Chem. C, 2015, 119, 2096–2104 CrossRef CAS.
- Y. Tang, N. Rong, F. Liu, M. Chu, H. Dong, Y. Zhang and P. Xiao, Appl. Surf. Sci., 2016, 361, 133–140 CrossRef CAS.
- J. Chouvin, C. Branci, J. Sarradin, J. Olivier-Fourcade, J. C. Jumas, B. Simon and P. Biensan, J. Power Sources, 1999, 81, 277–281 CrossRef.
- M. T. Sougrati, J. J. Arayamparambil, X. Liu, M. Mann, A. Slabon, L. Stievano and R. Dronskowski, Dalton Trans., 2018, 47, 10827 RSC.
- A. Eguia-Barrio, E. Castillo-Martinez, X. Liu, R. Dronskowski, M. Armand and T. Rojo, J. Mater. Chem. A, 2016, 4, 1608–1611 RSC.
- H. D. Schädler, L. Jäger and I. Senf, Z. Anorg. Allg. Chem., 1993, 619, 1115–1120 CrossRef.
- K. Dolabdjian, A. L. Görne, R. Dronskowski, M. Ströbele and H. J. Meyer, Dalton Trans., 2018, 47, 13378–13383 RSC.
- J. P. Allen, D. O. Scanlon, L. F. J. Piper and G. W. Watson, J. Mater. Chem. C, 2013, 1, 8194–8208 RSC.
- M. Neukirch, S. Tragl and H. J. Meyer, Inorg. Chem., 2006, 45, 8188–8193 CrossRef CAS PubMed.
- X. Liu, R. Dronskowski, R. Glaum and A. L. Tchougréeff, Z. Anorg. Allg. Chem., 2010, 636, 343–348 CrossRef CAS.
- (a) J. P. Allen, D. O. Scanlon, L. F. J. Piper and G. W. Watson, J. Mater. Chem. C, 2013, 1, 8194–8208 RSC; (b) M. Manikandan, T. Tanabe, P. Li, S. Ueda, G. V. Ramesh, R. Kodiyath, J. Wang, T. Hara, A. Dakshanamoorthy, S. Ishihara, K. Ariga, J. Ye, N. Umezawa and H. Abe, ACS Appl. Mater. Interfaces, 2014, 6, 3790–3793 CrossRef CAS PubMed; (c) S. Kaizra, B. Bellal, Y. Louafi and M. Trari, J. Saudi Chem. Soc., 2018, 22, 76–83 CrossRef CAS; (d) W. Xia, H. Wang, X. Zeng, J. Han, J. Zhu, M. Zhou and S. Wu, CrystEngComm, 2014, 16, 6841–6847 RSC; (e) M. Aslam, M. T. Qamar, S. Ali, A. U. Rehman, M. T. Soomro, I. Ahmed, I. M. I. Ismail and A. Hameed, J. Environ. Manage., 2018, 217, 805–814 CrossRef CAS PubMed; (f) L. Yang, J. Huang, L. Shi, L. Cao, W. Zhou, K. Chang, X. Meng, G. Liu, Y. Jie and J. Ye, Nano Energy, 2017, 36, 331–340 CrossRef CAS; (g) Y. Ogo, H. Hiramatsu, K. Nomura, H. Yanagi, T. Kamiya, M. Hirano and H. Hosono, Appl. Phys. Lett., 2008, 93, 032113 CrossRef.
- Q. Hou, M. Zhen, L. Liu, Y. Chen, F. Huang, S. Zhang, W. Li and M. Ju, Appl. Catal., B, 2018, 224, 183–193 CrossRef CAS.
- R. J. Müller, I. Kuznetsov, Y. Arbelo, M. Trottmann, C. S. Menoni, J. J. Rocca, G. R. Patzke and D. Bleiner, Anal. Chem., 2018, 90, 9234–9240 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9dt04752b |
| This journal is © The Royal Society of Chemistry 2020 |