Stereochemistry of coordination polyhedra vs. single ion magnetism in penta- and hexacoordinated Co(ii) complexes with tridentate rigid ligands†
Abstract
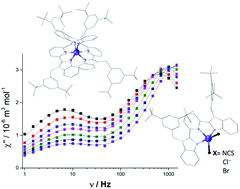
A tridentate ligand L (2,6-bis(1-(3,5-di-tert-butylbenzyl)-1H-benzimidazol-2-yl)pyridine) was synthesized and used for the preparation of three pentacoordinated Co(II) complexes of formula [Co(L)X2] (where X = NCS− for 1, X = Cl− for 2 and X = Br− for 3) and one ionic compound 4 ([Co(L)2]Br2·2CH3OH·H2O) containing a hexacoordinated Co(II) centre. Static magnetic data were analysed with respect to the spin (1–3) or the Griffith–Figgis (4) Hamiltonian. Ab initio calculations enable us to identify the positive axial magnetic anisotropy parameter D accompanied by a significant degree of rhombicity in the reported complexes. Also, magneto-structural correlation was outlined for this class of compounds. Moreover, all four compounds exhibit slow relaxation of magnetisation at an applied static magnetic field with either both low- and high-frequency relaxation channels (3) or a single high-frequency relaxation process (1, 2 and 4). The interplay between the stereochemistry of coordination polyhedra, magnetic anisotropy and the relaxation processes was investigated and discussed in detail.


 Please wait while we load your content...
Please wait while we load your content...