Abstract
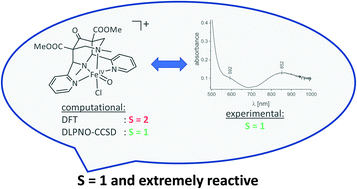
The iron(IV)oxido complex [(bispidine)FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(Cl)]+ is shown by experiment and high-level DLPNO-CCSD(T) quantum-chemical calculations to be an extremely short-lived and very reactive intermediate-spin (S = 1) species. At temperatures as low as −90 °C, it decays with a half-life of approx. two minutes, and this is the reason why, so far, it remained undetected and why it is extremely difficult to trap and fully characterize this interesting and extremely efficient oxidant. The large difference in reactivity between [(bispidine)FeIV
O(Cl)]+ is shown by experiment and high-level DLPNO-CCSD(T) quantum-chemical calculations to be an extremely short-lived and very reactive intermediate-spin (S = 1) species. At temperatures as low as −90 °C, it decays with a half-life of approx. two minutes, and this is the reason why, so far, it remained undetected and why it is extremely difficult to trap and fully characterize this interesting and extremely efficient oxidant. The large difference in reactivity between [(bispidine)FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(Cl)]+ and [(bispidine)FeIV
O(Cl)]+ and [(bispidine)FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(MeCN)]2+ (at least two orders of magnitude), while both oxido-iron(IV) complexes have very similar structures and an S = 1 electronic ground state, is presumably due to the large difference in the energy gap between the triplet and quintet electronic states. In presence of cyclohexane as substrate, [(bispidine)FeIV
O(MeCN)]2+ (at least two orders of magnitude), while both oxido-iron(IV) complexes have very similar structures and an S = 1 electronic ground state, is presumably due to the large difference in the energy gap between the triplet and quintet electronic states. In presence of cyclohexane as substrate, [(bispidine)FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(Cl)]+ oxidizes cyclohexane with a rate that is approx. 25 times faster than the self-decay of the oxidant, and selectively leads to chlorocyclohexane in moderate yield. The S = 1 electronic ground state of [(bispidine)FeIV
O(Cl)]+ oxidizes cyclohexane with a rate that is approx. 25 times faster than the self-decay of the oxidant, and selectively leads to chlorocyclohexane in moderate yield. The S = 1 electronic ground state of [(bispidine)FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(Cl)]+ and a relatively low gap to the S = 2 state (approx. 6 kJ mol−1vs. approx. 75 kJ mol−1 for [(bispidine)FeIV
O(Cl)]+ and a relatively low gap to the S = 2 state (approx. 6 kJ mol−1vs. approx. 75 kJ mol−1 for [(bispidine)FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(MeCN)]2+) is also predicted by DLPNO-CCSD(T) quantum-chemical calculations. The method used was benchmarked with a set of six ferryl complexes with experimentally known electronic ground states.
O(MeCN)]2+) is also predicted by DLPNO-CCSD(T) quantum-chemical calculations. The method used was benchmarked with a set of six ferryl complexes with experimentally known electronic ground states.
- This article is part of the themed collection: Inorganic Reaction Mechanisms


 Please wait while we load your content...
Please wait while we load your content...