 Open Access Article
Open Access ArticleNovel phthiocol-based organometallics with tridentate coordination motif and their unexpected cytotoxic behaviour†
Heiko
Geisler
 a,
Debora
Wernitznig
a,
Debora
Wernitznig
 a,
Michaela
Hejl
a,
Natalie
Gajic
a,
Michael A.
Jakupec
a,
Michaela
Hejl
a,
Natalie
Gajic
a,
Michael A.
Jakupec
 ab,
Wolfgang
Kandioller
ab,
Wolfgang
Kandioller
 *ab and
Bernhard K.
Keppler
*ab and
Bernhard K.
Keppler
 ab
ab
aUniversity of Vienna, Faculty of Chemistry, Institute of Inorganic Chemistry, Waehringer Str. 42, A-1090 Vienna, Austria. E-mail: wolfgang.kandioller@univie.ac.at; Tel: +43 1 4277 52609
bResearch Cluster “Translational Cancer Therapy Research”, University of Vienna, Waehringer Str. 42, A-1090 Vienna, Austria
First published on 17th January 2020
Abstract
Novel phthiocol-based organometallics with in situ formed tridentate N,O,O-coordination motif were established via three-component microwave assisted one-pot reaction. These complexes exhibited enhanced stability in aqueous solution compared to the parental compound KP2048 and showed unexpected cytotoxic behaviour and selectivity in 2D and 3D cell cultures.
Ruthenium arene complexes have shown promising anticancer activities in vitro and in vivo and numerous examples with different coordination motifs with various mono or bidentate ligand scaffolds, arenes and leaving groups have been reported in the literature.1–4 Attaching bioactive ligands to metal centres is an interesting approach for the development of metallodrugs with different modes of action compared to the ‘classical’ platinum-based anticancer agents.5 Quinones, especially 1,4-naphthoquinones, feature several intriguing characteristics, such as antibacterial, antiallergic, antifungal and antiviral, and thus were studied intensively over the last decades.6 The antitumor activity of naphthoquinones arises from the participation in cellular redox cycling and the generation of reactive oxygen species (ROS), which can lead to the oxidation of proteins, lipids, DNA or activate signalling pathways.6 We have recently shown that coordination of quinones (lapachol or phthiocol) with transition metals (Ru(II), Os(II) or Rh(III)) provided coordination compounds with enhanced cytotoxicity in several cancer cell lines.7,8 In particular, the phthiocol-based ruthenium complex 1 (KP2048; Fig. 1) showed promising results in vitro and in vivo. However, intraperitoneal treatment of mice with KP2048 led to severe side effects, such as stiff and swollen intestines, growth of the liver, spleen and stomach, due to the local extensive reactivity.7 UV-Vis measurements of compound 1 in phosphate-buffered saline (PBS) solution revealed that this complex is prone to decomposition under physiological conditions (see Fig. S28 and S29†). These findings explain the extensive reactivity of this species as the complex is hydrolysed quickly, followed by ligand cleavage.
Consequently, its lack of stability is responsible for its undesired local reactivity. Hence, the complex stability was improved by insertion of a pH-dependent leaving group coupled to poly(organo)phosphazenes.7 Thereby, an improved cellular uptake and accumulation into tumour cells could be achieved. Promising results in in vivo experiments substantiated the necessity of more stable leaving groups, in order to improve the complex stability and its activity in tumour cells. Within this work various primary amines (e.g., n-propylamine), secondary amines (morpholine, diisopropylamine), pyridines (pyridine, picoline), 1,3-diazoles (1H-imidazole, 1-methyl-1H-imidazole, 1H-benzimidazole) and 1,2-diazoles (1H-pyrazole (HPz), 1H-indazole (HInd), 4-methyl-1H-pyrazole (4-MeHPz), 4-amino-1H-pyrazole (4-NH2-HPz)9 and 6-amino-1H-indazole (6-NH2-HInd)) were attempted to replace the labile chloride leaving group.
The first experiments were performed according to literature procedures: firstly, the well-established synthesis of a disubstituted precursor complex followed by complexation with phthiocol L and a base (see Schemes S1 and S2†),10,11 and secondly, the use of silver salts to form the aqua complex, and introduction of the desired N-containing ligand.12 However, successful complexation was only observed with 1,2-diazoles. Unexpectedly, formation of a new tridentate ligand scaffold coordinated to the organometallic fragment took place, where a hemiaminal bond connects the naphthoquinone and the N1 nitrogen of the azole moiety (see Scheme 1). Thus, these complexes feature an additional five-membered ring between the metal centre, the oxygen (O1), the quaternary carbon (C1) and the two nitrogens of the 1,2-diazole moiety. Furthermore, these complexes exhibit two chirality centres, one at the C1 carbon which connects the pyrazole ring, the metal centre and the naphthoquinone and the second one at the metal centre itself. Due to the prevalent structure of these complexes, only two enantiomers can be formed (RC1,RRu and SC1,SRu). Moreover, this in situ formation also appeared in the case of Os(II) as metal centre; however, Rh(III) and Ir(III) did not provide complexes with this tridentate coordination motif. After the preferential formation of a tridentate ligand was observed, it was possible to establish a straightforward three-component microwave synthesis. The improved stability due to the lack of a labile chlorido leaving group allows purification by column chromatography. All complexes (1a–e, 2a–c) could be synthesised via this one-pot reaction, where the respective dimer ([RuCl2(p-cymene)]213 or [OsCl2(p-cymene)]2),14 phthiocol (L)15,16 and 1,2-diazole (HPz, HInd, 4MeHPz, 4-NH2-HPz,96-NH2-HInd) were stirred in the presence of a base (NaOMe or NEt3) under microwave irradiation for 6–12 minutes at 50–60 °C and purified via column chromatography using a ternary eluent system (EtOAc/n-hex/NEt3 or EtOAc/MeOH/NH4OH) in moderate to good yields (41–84%).
Formation and purity of the complexes were confirmed by 2D-NMR spectroscopy and elemental analysis (see Fig. S1–17†). The 13C-NMR spectra of the complexes contain a signal around 90 ppm with low intensity, which approves the hemiaminal bond of the tridentate ligand. Additionally, single crystals of all complexes were obtained by vapour-diffusion (1c, d, 2a and 2c) or liquid-liquid-diffusion (1a,b and 2b) from dichloromethane/diethyl ether or ethyl acetate/n-hexane (see Fig. S18–S27 and Tables S1–S17†). Complexes 1b, 1c and 2b crystallised in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Complexes 1a (Fig. 2), 1d, 1e, 2a and 2c crystallise in the monoclinic space groups C2/c, P21/n and P21/c. Complexes containing tridentate ligands feature decreased bond lengths between O1 and the metal centre (0.05–0.090 Å), compared to compound 1, whereas the bond length of O2–M elongated slightly (max. 0.04 Å). The bond length between the metal centre and the chlorido leaving group of complex 1 is 2.403 Å, which is considerably longer than the nitrogen-metal bond (2.078–2.116 Å) for these novel complexes.
. Complexes 1a (Fig. 2), 1d, 1e, 2a and 2c crystallise in the monoclinic space groups C2/c, P21/n and P21/c. Complexes containing tridentate ligands feature decreased bond lengths between O1 and the metal centre (0.05–0.090 Å), compared to compound 1, whereas the bond length of O2–M elongated slightly (max. 0.04 Å). The bond length between the metal centre and the chlorido leaving group of complex 1 is 2.403 Å, which is considerably longer than the nitrogen-metal bond (2.078–2.116 Å) for these novel complexes.
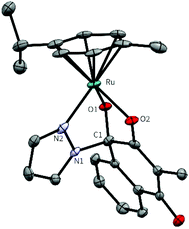 | ||
| Fig. 2 Molecular structure of complex 1a (M = Ru, R = HPz) at a 50% probability level. Hydrogens and solvent molecules were omitted for clarity. | ||
The stability of the organometallic complexes in aqueous solution (PBS, pH = 7.4, 25 °C) was determined by UV/Vis spectroscopy. Due to their poor solubility in aqueous media, 1% DMF (1) or DMSO (1a–e, 2a–c) was used as solubilizer. Minor changes in the absorption curve of compound 1 and subsequent comparison to the free ligand (L) spectrum revealed a rapid degradation of complex 1 in aqueous solution (see Fig. S28 and S29†). Nevertheless, complexes 1a–2c possess improved stability at pH 7.4, which is indicated by only small changes of the respective absorption curves over time (see Fig. S30–35†) compared to the immediate cleavage of KP2048 under these conditions. Due to presence of the hemiaminal structural feature, experiments were performed to elucidate the impact of the pH value on the aquation rate of these complexes. However, only minor changes with regard on the reaction kinetics were observed for complex 1a under these conditions (pH 5.8–7.9, see Fig. 3 and Fig. S36–S40†).
 | ||
| Fig. 3 Left: Absorption curves of compound 1a over 48 h in PBS (pH = 7.2; 25 °C). Right: Absorption vs. time at 363 nm at different pH values (5.8–7.9). | ||
The cytotoxic behaviour of the complexes 1a–2c was examined with the obtained mixture of stereoisomers (RC1, RM and SC1, SM) by the colorimetric MTT assay in the human cancer cell lines CH1/PA-1 (ovarian teratocarcinoma), SW480 (colon carcinoma) and A549 (non-small cell lung carcinoma). The building blocks of the complexes, phthiocol L, azoles (see Table S18†) and dimeric metal precursors showed no relevant cytotoxic activities. Compound 1 with chlorido as leaving group exhibited increased cytotoxicity compared to the free ligand phthiocol L. However, introduction of the tridentate N,O,O coordination motif dramatically changed the cytotoxic properties of the complexes. Overall the developed complexes exhibited cytotoxic potencies down to the low nanomolar range in SW480 (IC50 values: 0.057–5.5 μM) and A549 (IC50 values: 0.91–47 μM) cancer cells, with ruthenium compounds being slightly more active than their osmium analogues (Table 1, Fig. S41 and S42†).
| IC50/μM | |||
|---|---|---|---|
| A549 | SW480 | CH1/PA-1 | |
| L | 210 ± 32 | 116 ± 37 | 129 ± 29 |
| 1 (KP2048) 7 | 47 ± 4 | 15 ± 3 | 31 ± 10 |
| 1a | 1.2 ± 0.2 | 0.094 ± 0.031 | >50 |
| 1b | 0.91 ± 0.10 | 0.057 ± 0.008 | 40 ± 4 |
| 1c | 2.1 ± 0.6 | 0.17 ± 0.04 | 119 ± 25 |
| 1d | 47 ± 10 | 5.5 ± 1.5 | 62 ± 4 |
| 1e | 5.4 ± 1.0 | 0.62 ± 0.06 | 102 ± 16 |
| 2a | 7.4 ± 0.3 | 0.26 ± 0.03 | >100 |
| 2b | 3.2 ± 0.3 | 0.16 ± 0.03 | 61 ± 7 |
| 2c | 13 ± 3 | 0.49 ± 0.13 | 117 ± 3 |
| Cisplatin 17 | 6.2 ± 1.2 | 3.3 ± 0.2 | 0.077 ± 0.006 |
| KP1339/BOLD-100 18 | 156 ± 11 | 88 ± 19 | 62 ± 9 |
Conversely, cytotoxic potencies of these compounds are surprisingly reduced in the cancer cell line CH1/PA-1 (IC50 values: 40–119 μM), although these cells have shown to be highly sensitive to many coordination compounds of, e.g., Ru(II), Ru(III), Os(II), Rh(III), Au(I), Ag(I), Cu(II), Pt(II), Pt(IV) and Fe(II).7,8,11,19–22 In contrast, the activity in the rather insensitive SW480 cells of ruthenium compounds 1a–c is increased by at least two orders of magnitude compared to the CH1/PA-1 cell line. These results may portend to a pronounced selectivity for SW480 cells. Amino functionalised ruthenium arene complexes 1e, 1d revealed improved aqueous solubility; however, cytotoxicity was lowered up to 60 times depending on the cell line. Therefore, it can be assumed that the antiproliferative activity critically depends on the azole moiety. The cytotoxic potencies of 1a,b in the cancer cell line SW480 are comparable with the currently most active organoruthenium complexes reported by Süss-Fink and co-workers (with IC50 values down to 30 nM in A2780 and A2780cisR cells).23,24 The only other reported Ru(II) arene complexes bearing a tridentate ligand scaffold were based on a N,N,N coordination motif (diethylenetriamine) and found to be nearly inactive. It was assumed that this behaviour arises from the lack of a labile leaving group, which leads to inertness against ligand exchange reactions such as aquation and therefore prevents biomolecule interactions.25
The compounds were also tested for their anticancer activity in four different human cancer cell lines grown as multicellular spheroids with an exposure time of 96 h (Fig. 4). These 3D models provide more information about the cytotoxic behaviour of the compounds, since spheroids are able to mimic the main properties of human solid tumours.26 It is known from the literature that the enhanced in vitro activity of some metal-based compounds in 2D monolayers is dramatically reduced when the experiments are performed using 3D models.27 They displayed varying cytotoxic potencies depending on the cell line. Interestingly, all compounds were very active in the usually more ‘resistant’ cell lines A-549, HCT-15 and HCT-116. The surprisingly low IC50 values obtained in the three-dimensional in vitro model are listed in Table 2. These results are in good agreement with the obtained 2D data and confirm the unexpected activity in more chemo-resistant human cancer cell lines. Further studies are necessary and ongoing to explain the mechanisms underlying the resistance of the CH1/PA-1 cell line to these compound class, which lead to the major differences in the IC50 values compared to the other three cell lines. Additionally, the CH1/PA-1 cell line might represent a valuable tool, which might support the understanding of the actual mode of action of this compound class.
 | ||
| Fig. 4 Representative images of HCT-15 and HCT-116 multicellular spheroids treated with novel complexes at about the respective IC50 for 96 h, compared to untreated controls. | ||
| IC50/μM | ||||
|---|---|---|---|---|
| A549 | HCT-15 | HCT-116 | CH1/PA-1 | |
| 1a | 4.6 ± 3.1 | 0.97 ± 0.43 | 0.67 ± 0.27 | 50 ± 8 |
| 1b | 5.1 ± 3.1 | 0.74 ± 0.45 | 0.95 ± 0.28 | 118 ± 9 |
| 1c | 1.4 ± 0.2 | 0.99 ± 0.09 | 1.4 ± 0.3 | 95 ± 10 |
| 2a | 15 ± 1 | 1.8 ± 0.5 | 21 ± 3 | >400 |
| 2b | 8.2 ± 1.2 | 1.6 ± 0.5 | 15 ± 1 | 135 ± 10 |
| 2c | 17 ± 2 | 4.5 ± 1.1 | 31 ± 4 | >400 |
To the best of our knowledge we report on the first examples of highly cytotoxic tridentate N,O,O-coordinated M(arene) complexes, which were synthesised via a three-component one-pot reaction under microwave conditions. The formation and purity of the compounds was confirmed by standard analytical methods. The improved stability compared to the parental complex KP2048 was proved by UV/Vis measurements under physiologically relevant conditions. Besides the enhanced stability, the introduction of the 1,2-diazole moiety allows further fine-tuning of pharmacokinetic properties, due to the broad range of feasible modifications at this site. It was found that this compound class, although designed as prodrugs for KP2048, is not activated by transformation to this species under acidic conditions and this reflects in a different cytotoxicity profile in vitro. The complexes displayed a remarkably high activity in the usually rather insensitive human cancer cell lines SW480 and A549 with IC50 values down to 57 nM, which is in the same range as the most potent organoruthenium complexes reported so far. Surprisingly, these organometallics show no relevant cytotoxicity in the chemo-sensitive cell line CH1/PA-1. The same trend was observed in a variety of multicellular tumour spheroid models, where also pronounced cytotoxic activities were observed in those grown from more chemo-resistant cell lines.
However, further experiments are necessary and currently ongoing to clarify the observed highly unexpected cytotoxic behaviour.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. K. Yan, M. Melchart, A. Habtemariam and P. J. Sadler, Chem. Commun., 2005, 4764–4776, 10.1039/b508531b.
- G. Suss-Fink, Dalton Trans., 2010, 39, 1673–1688 RSC.
- L. Zeng, P. Gupta, Y. Chen, E. Wang, L. Ji, H. Chao and Z. S. Chen, Chem. Soc. Rev., 2017, 46, 5771–5804 RSC.
- T. Bugarcic, A. Habtemariam, R. J. Deeth, F. P. Fabbiani, S. Parsons and P. J. Sadler, Inorg. Chem., 2009, 48, 9444–9453 CrossRef CAS PubMed.
- K. J. Kilpin and P. J. Dyson, Chem. Sci., 2013, 4, 1410–1419 RSC.
- K. W. Wellington, RSC Adv., 2015, 5, 20309–20338 RSC.
- C. M. Hackl, B. Schoenhacker-Alte, M. H. M. Klose, H. Henke, M. S. Legina, M. A. Jakupec, W. Berger, B. K. Keppler, O. Brüggemann, I. Teasdale, P. Heffeter and W. Kandioller, Dalton Trans., 2017, 46, 12114–12124 RSC.
- W. Kandioller, E. Balsano, S. M. Meier, U. Jungwirth, S. Göschl, A. Roller, M. A. Jakupec, W. Berger, B. K. Keppler and C. G. Hartinger, Chem. Commun., 2013, 49, 3348–3350 RSC.
- C. Maccallini, M. Di Matteo, D. Vullo, A. Ammazzalorso, S. Carradori, B. De Filippis, M. Fantacuzzi, L. Giampietro, A. Pandolfi, C. T. Supuran and R. Amoroso, ChemMedChem, 2016, 11, 1695–1699 CrossRef CAS PubMed.
- M. Schmidlehner, P.-S. Kuhn, C. M. Hackl, A. Roller, W. Kandioller and B. K. Keppler, J. Organomet. Chem., 2014, 772–773, 93–99 CrossRef CAS.
- C. M. Hackl, M. S. Legina, V. Pichler, M. Schmidlehner, A. Roller, O. Domotor, E. A. Enyedy, M. A. Jakupec, W. Kandioller and B. K. Keppler, Chemistry, 2016, 22, 17269–17281 CrossRef CAS.
- C. A. Riedl, M. Hejl, M. H. M. Klose, A. Roller, M. A. Jakupec, W. Kandioller and B. K. Keppler, Dalton Trans., 2018, 47, 4625–4638 RSC.
- S. B. Jensen, S. J. Rodger and M. D. Spicer, J. Organomet. Chem., 1998, 556, 151–158 CrossRef CAS.
- W. A. Kiel, R. G. Ball and W. A. G. Graham, J. Organomet. Chem., 1990, 383, 481–496 CrossRef CAS.
- L. Kathawate, S. P. Gejji, S. D. Yeole, P. L. Verma, V. G. Puranik and S. Salunke-Gawali, J. Mol. Struct., 2015, 1088, 56–63 CrossRef CAS.
- R. Zhu, L. Xing, X. Wang, C. Cheng, B. Liu and Y. Hu, Synlett, 2007, 2267–2271 CAS.
- H. P. Varbanov, S. Goschl, P. Heffeter, S. Theiner, A. Roller, F. Jensen, M. A. Jakupec, W. Berger, M. Galanski and B. K. Keppler, J. Med. Chem., 2014, 57, 6751–6764 CrossRef CAS PubMed.
- P. S. Kuhn, V. Pichler, A. Roller, M. Hejl, M. A. Jakupec, W. Kandioller and B. K. Keppler, Dalton Trans., 2015, 44, 659–668 RSC.
- H. P. Varbanov, M. A. Jakupec, A. Roller, F. Jensen, M. Galanski and B. K. Keppler, J. Med. Chem., 2013, 56, 330–344 CrossRef CAS PubMed.
- M. F. Primik, S. Goschl, M. A. Jakupec, A. Roller, B. K. Keppler and V. B. Arion, Inorg. Chem., 2010, 49, 11084–11095 CrossRef CAS PubMed.
- M. J. McKeage, P. Papathanasiou, G. Salem, A. Sjaarda, G. F. Swiegers, P. Waring and S. B. Wild, Met.-Based Drugs, 1998, 5, 217–223 CrossRef CAS PubMed.
- A. Houlton, R. M. G. Roberts and J. Silver, J. Organomet. Chem., 1991, 418, 107–112 CrossRef CAS.
- A. P. Basto, J. Muller, R. Rubbiani, D. Stibal, F. Giannini, G. Suss-Fink, V. Balmer, A. Hemphill, G. Gasser and J. Furrer, Antimicrob. Agents Chemother., 2017, 61 CrossRef CAS PubMed.
- F. Giannini, J. Furrer, A. F. Ibao, G. Suss-Fink, B. Therrien, O. Zava, M. Baquie, P. J. Dyson and P. Stepnicka, J. Biol. Inorg. Chem., 2012, 17, 951–960 CrossRef CAS PubMed.
- M. V. Babak, S. M. Meier, A. A. Legin, M. S. Adib Razavi, A. Roller, M. A. Jakupec, B. K. Keppler and C. G. Hartinger, Chemistry, 2013, 19, 4308–4318 CrossRef CAS PubMed.
- A. S. Nunes, A. S. Barros, E. C. Costa, A. F. Moreira and I. J. Correia, Biotechnol. Bioeng., 2019, 116, 206–226 CrossRef CAS PubMed.
- E. Schreiber-Brynzak, E. Klapproth, C. Unger, I. Lichtscheidl-Schultz, S. Goschl, S. Schweighofer, R. Trondl, H. Dolznig, M. A. Jakupec and B. K. Keppler, Invest. New Drugs, 2015, 33, 835–847 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses of the complexes, NMR characterization, stability studies via UV/Vis, crystallographic data. CCDC 1955180–1955187. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9dt04462k |
| This journal is © The Royal Society of Chemistry 2020 |


