Rh–M (M: Co, Cu, and Fe) nanoclusters as highly efficient and durable catalysts for the methanolysis of ammonia borane
Abstract
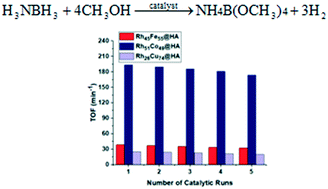
Herein, we report the preparation, characterization, and employment of hydroxyapatite-supported rhodium-based Rh–M (RhCo, RhCu, and RhFe) nanoclusters as cost-effective, highly efficient and reusable catalysts for hydrogen evolution from ammonia borane methanolysis. Rh–M@HA nanoclusters are formed by in situ reduction of hydroxyapatite-supported related metal cations during ammonia borane methanolysis after ion-exchange. The Rh–M@HA nanoclusters are easily obtained as very stable solids and identified by some advanced analytical techniques like ICP-OES, BET, TEM, XPS, and XRD. After experimental studies, they are found as highly isolable, redispersible, and reusable nanocatalysts to evolve hydrogen from ammonia borane methanolysis under mild conditions. From these three catalysts, the Rh51Co49@HA nanoclusters provide the highest turnover frequency (TOF) value of 193.7 min−1 while the Rh26Cu74@HA and Rh45Fe55@HA nanoclusters have TOF values of 25.1 and 38.7 min−1, respectively, in ammonia borane methanolysis at 25 ± 0.1 °C. The apparent activation energies (Ea) of the Rh51Co49@HA, Rh26Cu74@HA, and Rh45Fe55@HA nanoclusters for the same reaction are calculated to be 52.7, 59.7, and 54.1 kJ mol−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...