Insights into efficient removal of gaseous p-xylene using cerium-doped ZnO nanoparticles through photocatalytic oxidation†
Abstract
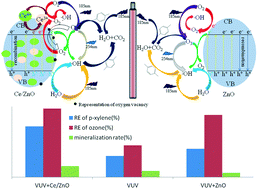
Volatile organic compounds (VOCs) are considered to be the main cause of air pollution and should be controlled strictly. Vacuum ultraviolet combined with cerium (Ce)-doped ZnO as the catalyst was investigated for the removal of para-xylene (p-xylene). Based on scanning electron microscopy, X-ray photoelectron spectroscopy and X-ray diffraction analysis, the particle size decreased after cerium in the form of Ce3+ and Ce4+ was doped into the ZnO crystal structure. An improved photocatalytic activity (higher p-xylene removal efficiency) was obtained at 0.96% Ce doping, 351 °C calcination temperature, and 339 min calcination duration. Based on the investigation of process parameters, the removal efficiency of p-xylene reached 100% with an initial concentration of 150 mg m−3, a relative humidity of 50%, and a residence time of 60 s. The ozone utilization extent was 96%, suggesting that the remaining ozone after the photocatalysis of p-xylene could be well controlled. Four different kinetic rate equations were used, suggesting that p-xylene and water molecules competed for the same active site. Mechanism analysis suggested that four types of oxidation contributed jointly to the removal of p-xylene and they followed this order: oxidation by the reactive species (RS, mainly ˙OH, O3 and O(1D)) > oxidation by the synergistic interactions among photolysis, RS and h+ > transformation by direct photolysis > oxidation by h+.

 Please wait while we load your content...
Please wait while we load your content...