Palladium-catalysed Heck-type alkenylation reactions in the synthesis of quinolines. Mechanistic insights and recent applications
Abstract
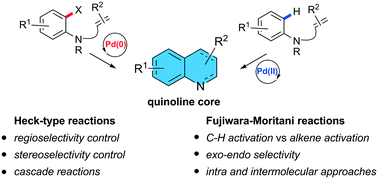
Quinoline and quinolone cores are present in a wide variety of pharmaceuticals, agrochemicals and materials as well as in ligands/catalysts in asymmetric synthesis. Transition metal catalysed based approaches have played a pivotal role in the development of the catalytic methods for their synthesis. This review presents recent developments on the palladium-catalysed Mizoroki–Heck reaction and its dehydrogenative variant (the Fujiwara–Moritani reaction), a C–H activation reaction that does not require the use of prefunctionalized coupling patterns for the synthesis of quinoline frameworks. The mechanistic understanding of both types of reactions and how the different reaction conditions affect the outcome and the regioselectivity for the synthesis of the quinoline core, which is crucial to achieve target-oriented synthesis successfully, is discussed through selected examples.


 Please wait while we load your content...
Please wait while we load your content...