Macroporous polymer resin with conjugated side-chains: an efficient Ag nanoparticle support for preparing a photocatalyst†
Abstract
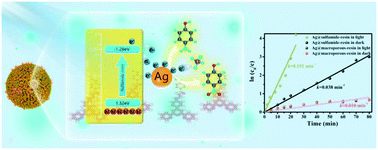
Silver nanoparticles (Ag NPs), widely used as photocatalysts, exhibit low photocatalytic activity due to their serious aggregation and difficulty in separation for recycling. To solve these problems, a highly efficient and stable photocatalysis system was designed by immobilizing Ag NPs on a macroporous polymer resin with conjugated side-chains (denoted as Ag@sulfamide-resin). A macroporous polymer resin with conjugated side-chains (named sulfamide-resin) was constructed by grafting 4′-(p-ethanediamine phenyl)-2,2′:6′,2′′-terpyridine (TE) to polystyrene-based macroporous resin via sulfamide bond formation. The photocatalytic activity of Ag@sulfamide-resin was evaluated via the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) as a model reaction. Ag@sulfamide-resin exhibits excellent photocatalytic activity and stability, and the catalytic activity of Ag@sulfamide-resin under light irradiation was found to be 3.97 and 15.1 times those of Ag@sulfamide-resin in the dark and Ag@macroporous-resin under light irradiation, respectively. The higher photocatalytic activity was attributed to the fact that sulfamide resin can absorb broad wavelength light and easily transfer photogenerated electrons to Ag NPs; the abundant “N” atoms in this resin can act as anchoring sites for Ag NPs to prevent their aggregation and loss; and Ag NPs display smaller sizes of 2–3 nm. The macroporous polymer resin with conjugated side-chains may be a prospective material as a support for designing an efficient photocatalyst.


 Please wait while we load your content...
Please wait while we load your content...