Enhancing hydrogen evolution reaction through modulating electronic structure of self-supported NiFe LDH†
Abstract
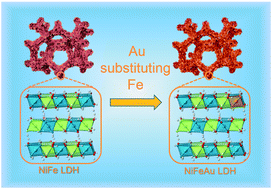
NiFe-layered double hydroxide (NiFe LDH), as an efficient oxygen evolution reaction (OER) electrocatalyst, has emerged as a promising electrocatalyst for catalyzing overall water splitting in alkaline electrolyte. However, its sluggish hydrogen evolution reaction (HER) process greatly limits its practical applications. Here, we develop a NiFeAu LDH with Au atoms partially substituting Fe atoms via a simple one-step hydrothermal process to improve its HER performance. Experimental results reveal that electronegative Au atoms change the electronic structure of the NiFe LDH, resulting in enhanced conductivity and facile electron transfer. The NiFeAu LDH affords superior HER activities with a low overpotential of 89 mV at 10 mA cm−2, and even exhibits a low overpotential of 192 mV at a high current density of 100 mA cm−2. Furthermore, its OER performance is also greatly improved, with overpotentials of 181 mV and 267 mV to achieve 10 mA cm−2 and 100 mA cm−2. Consequently, when the NiFeAu LDH is used as both a cathode and an anode for overall water splitting, only 1.57 V battery voltage is needed to reach 10 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...