Efficient synthesis of chiral γ-aminobutyric esters via direct rhodium-catalysed enantioselective hydroaminomethylation of acrylates†
Abstract
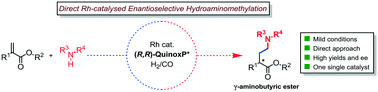
The successful rhodium catalysed asymmetric intermolecular hydroaminomethylation (HAM) of alkenes using a single catalyst bearing the (R,R)-QuinoxP* ligand is reported. The HAM of α-alkyl acrylates is revealed to be an efficient tool for the regio- and enantioselective synthesis of γ-amino esters with ees up to 86%. HPNMR experiments provided insights into the reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...