Open Access Article
Open Access ArticleSteroidal supramolecular metallogels
Riikka
Kuosmanen
,
Kari
Rissanen
 * and
Elina
Sievänen
* and
Elina
Sievänen
 *
*
Department of Chemistry, University of Jyväskylä, P.O. Box 35, FI-40014, Finland. E-mail: kari.t.rissanen@jyu.fi; elina.i.sievanen@jyu.fi
First published on 4th March 2020
Abstract
The review deals with an expanding number of steroidal compounds that are capable of forming a metallogel providing a multitude of novel materials rich in their properties. The future of steroidal metallogels holds a myriad of potential applications as new intelligent materials. Detection of potentially harmful compounds without expensive instrumentation, entrapment of environmentally hazardous substances, and sensitive and selective nanomaterials represent only a few of these potential applications. This article reviews the design, synthesis, characterization, and applications of steroidal metallogels.
1 Introduction
The first steroidal supramolecular metallogel was observed in 1913.1 Since then, a myriad of steroidal as well as other supramolecular (metallo)gels have been discovered. Although supramolecular gels have been utilized in various branches of science, supramolecular metallogels are a relatively new area in the field. It has gained increasing interest only in the 21st century.Multiple biologically significant natural products belong to the group of steroids. Animal steroids, such as cholesterol, bile acids, corticosteroids, and mammalian sex hormones, contain a cholesterol-derived backbone. Cholesterol itself is essential for the function and structure of animal cell membranes, where it effects on the membrane fluidity, microdomain structure, and permeability. It acts as a precursor for steroid hormones, bile acids, vitamin D, and lipoproteins, but also correlates with harmful conditions, such as cardiovascular disease, atherosclerosis, and gallstones.2 The chemical structure of plant sterols is similar to that of cholesterol, differing mainly in the side chain. Similarly to cholesterol in animal cells, the phytosterols in plants impact on the structure and function of the cell membranes by controlling their fluidity and permeability. The most common representatives of phytosterols are sitosterol, stigmasterol, and campesterol.3
Gels can be found everywhere in our daily life; hair gels, toothpaste, and soft contact lenses represent common examples of everyday applications of gels. Gels are generally considered to comprise of an elastic cross-linked network and the entrapped liquid. They have a solid-like rheology, although they mainly consist of liquid; typically gels contain 99% (w/v) of liquid, while the rest is the gelator. In supramolecular gels the low molecular weight gelators (LMWGs) self-assemble via non-covalent interactions (e.g., hydrogen bonds, π–π stacking, metal coordination, electrostatic, van der Waals, or dipole–dipole interactions) to form the network that is then capable of entrapping the liquid.4
Depending on the nature of the liquid, gels can be divided in organogels (organic solvent) and hydrogels (water).4 Gelator–gelator and gelator–solvent interactions play important roles in gel formation,4a which is why molecular level interactions need to be considered in correlating the gelator behavior with a given solvent. Prediction of gelation properties is thus challenging and comprehensive solvent screening tests are often required. In a typical gelation experiment a solution containing the gelator is heated above Tgel and the resulting solution allowed to slowly cool. The material is defined as a gel if upon inversion it is able to support its own weight. In some cases gelation can be brought up by ultrasound treatment or mechanical shaking.4b
Supramolecular gels have innumerable applications in different fields ranging from regenerative medicine and tissue-engineering to nanoelectronics.5,6 They can, for example, be utilized as long-term releasers of cancer drugs applied locally after tumor removal,7 as moulds for porous materials,8 or in cation and anion recognition.9
Supramolecular gels comprise an intriguing and a very contemporary research field owing to the challenges in defining the structure of the molecular network and the properties of the resulting gels. Over the last few decades, NMR, SEM, XRD, and other techniques have developed tremendously, giving new ways to study gels and their properties.4b,10 More elaborate liquid and solid state NMR techniques have enabled the study of the gelator molecules and gels in more detail in their native forms. Besides NMR spectroscopy, electron microscopy and powder X-ray diffraction may give insight into the organization of the fibrous nanostructures induced by the gelator molecules. High resolution pictures of xerogels and wet gels can be obtained with the help of SEM and cryo-SEM, respectively. By using field emission SEM (FE-SEM) ultra-clear, less electrostatically distorted images of the self-assembled nanostructures in the gels can be achieved.11 Furthermore, powder X-ray diffraction (PXRD) of the xerogels has been used to provide information on the solid-state-like organization of the gelator molecules.12 The rheological properties of the gels reveal, for example, how different stimuli change solid and fluid mechanics of the gels.10 Furthermore, UV-vis and fluorescence titrations,9b impedance spectroscopy,9b and differential scanning calorimetry (DSC)11 have been utilized in the research of supramolecular gels.
In metallogels metal ions take part in the process of self-assembly that leads to gelation. The metal ions may act as functional or simply coordinating entities,4b,10 being part of or auxiliary to the gelator. In the latter case, metal ions (or a mixture of metal ions) are added as a solution to the gel or to a solution containing the gelator.13a,14 The properties of metal ions, such as luminescence, open up a totally new field of applications for the gels,13 and for example the luminescent properties of the lanthanide-containing metallogels have been widely studied.15 Compared to traditional supramolecular gels, metallogels are able to respond to a wider range of physical and chemical stimuli. As a result, metallogels responding to multiple stimuli in intelligent materials may be part of our everyday lives in the future.
In metallogels, such as in supramolecular gels in general, self-assembly is a hierarchical process (Fig. 1). At first molecules form pairs, which then assemble into oligomers. Polymers are constructed from oligomers in the second phase. When the polymers interweave, thicker fibres are formed. These interweaved polymer fibres interact with each other via non-covalent interactions to give rise to the 3D networks, which are capable of immobilizing the solvent.13 The properties of the metallogels in particular, may be controlled or regulated by two principal methods: by selecting the metal-ion or by introducing changes in the structure of the gelator.10,13b
 | ||
| Fig. 1 Hierarchical self-assembly resulting in a supramolecular gel.10 | ||
Metallogels may form either by metal–ligand coordination upon addition of metal ions or by immobilization of solvent molecules by metal complexes. Depending on the way in which the metal-ions are incorporated into the gel network, the nature of the self-assembly process may vary. The metal to ligand ratio is defined by the properties of both the metal ion and the ligand. Labile metal ions tend to form complexes containing one metal-ion and several ligands. Ligands can adopt different coordination geometries, and in some cases the interactions with metal ions can be reversible. Typically in these cases the gel forming network arises from hydrogen bonds, van der Waals-forces, etc. Metal ions are also able to connect gelator molecules to each other, forming long chains. The formation of the 3D network in this case is based on metal coordination and is likely to be irreversible.10,13b
Metallogels have a tremendous amount of potential applications ranging from medicine to storage of information.10,13a,b,14 Metallogels, as other supramolecular gels, can be utilized as moulds in preparation of various materials. Also steroid-derived metallogels have been widely used for this purpose. Ln(III)-cholate gels, for example, have been applied as moulds in nanoparticle and nanotube preparation.11,16c,d,17 Calcium cholate hydrogels, which were utilized as moulds for palladium nanoparticle synthesis and further as reaction media, were even observed to possess remarkable catalytical properties.18 A lanthanide-containing bile acid-based hydrogel as a template enabled the in situ synthesis of lanthanide trifluoride nanoparticles even at room temperature.19 Ln(III) cholate gels have also been applied in detection of a multitude of analytes.20 Additionally, Cu(II) lithocholate hydrogel was used as a template for the preparation of CuS nanoparticles, which were successfully used in detecting ssDNA.16a One of the many future applications of metallogels, steroidal ones included, may be the separation of toxic dyes from wastewaters.21 Metallogels may also find use in electronic devices. For example, white light emitting materials, such as a cholate–lanthanide hydrogel,22 are important in manufacturing displays.
2 Steroidal metallogels
2.1 Ferrocene derivatives
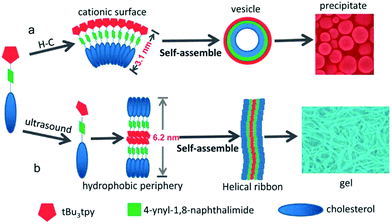
Conjugates of ferrocene and steroids were found to self-assemble into vesicles in oxidized form in the 1990s for the first time.23 Generally ferrocene conjugates have been observed to form gels poorly because of the non-polar nature of ferrocene.24 Ferrocene derivatives belong to the ALS-type of compounds. ALS-compounds consist of an aromatic part (A), which is connected with a (carbon) chain (L) to a steroid (S) (Fig. 2). The steroid molecule is typically cholesterol.12,25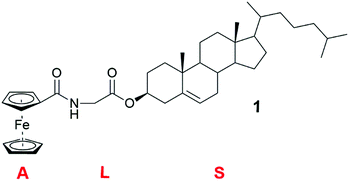 | ||
| Fig. 2 Cholesteryl glycinate ferrocenyl amide (compound 1).12 | ||
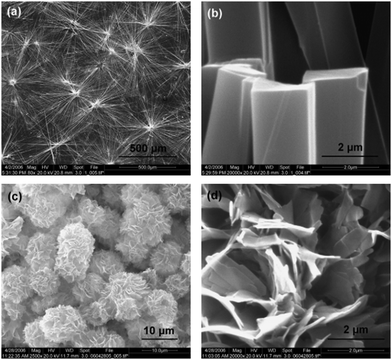
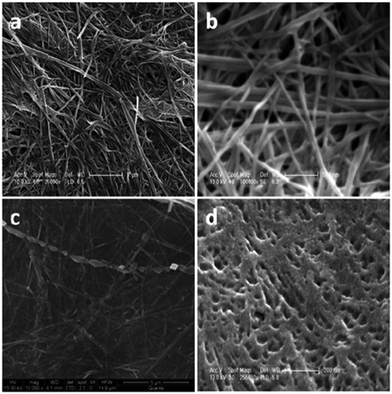
Cholesteryl glycinate ferrocenylamide (Fig. 2, compound 1) was observed to form a gel in EtOAc faster and in lower concentration than, for example, in alcohols.12 In the SEM images of the xerogel samples of compound 1 divergent structures were detected, depending on whether the gel had been formed in EtOAc or in 1,5-pentanediol (Fig. 3). The xerogel structures obtained from EtOAc resembled rod-like prisms (Fig. 3a and b), which had assembled into ball-shaped entities similar to the fluffy seeds of dandelions. The xerogel structures obtained from 1,5-pentanediol, however, possessed sheet-like structures assembled into burdock-shaped balls (Fig. 3c and d). Interestingly, in PXRD perfect lamellar structures were observed for the powder of 1 as well as for the powder of the xerogel of 1 obtained from EtOAc. Based on the XRD it was suggested that the cholesterol moieties were pointing to the sides, whereas the ferrocene moieties were stacked on top of each other.
When the alkyl chain of the diamino linker between the steroid and the ferrocene in ALS-compounds 2–5 (Fig. 4) was lengthened, the gelation ability was observed to weaken.25a Compound 2 with one methylene group in the chain was found to act as a supergelator, forming a gel under 1% (w/v), in cyclohexane. Compound 3 with two methylene groups formed fewer gels than 2, and compounds 4 and 5 with three and four methylene groups, respectively, did not form gels at all. In addition to interaction between the ferrocene and cholesterol moieties, hydrogen bonding between linker chains was observed to play an important role in gelation on the grounds of FTIR- and both temperature- and concentration-depended 1H NMR-measurements.
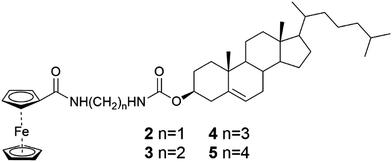 | ||
| Fig. 4 Cholesterol ferrocenyl derivatives representing the ALS type.25a | ||
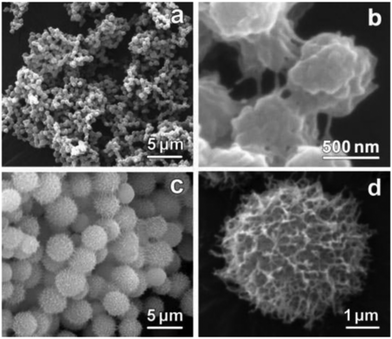
The SEM-images of the xerogels of compounds 2 and 3 (Fig. 5) showed similar ball-shaped structures than detected for the xerogels of compound 1.12 Compound 2 possessed different morphologies depending on the concentration: at lower concentration the gel network resembled a spider's web, whereas at higher concentration balls and fibers were observed.25a At the highest concentration studied only spherical objects resembling cotton balls were detected. Compared to compound 2 compound 3 formed larger ball-shaped structures, which resembled burdocks. In both cases the ball-shapes were constructed from nanofibers.
In a series of ferrocene compounds 6–8 representing the LS2 type (Fig. 6), where two steroidal moieties are connected via a chain bearing the ferrocenyl part, the variations in the hydrogen-bonding capabilities were speculated to cause the different gelation properties of the compounds.25b
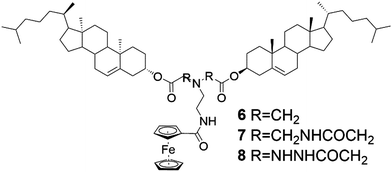 | ||
| Fig. 6 Cholesterol ferrocenyl derivatives representing the LS2 type.25b | ||
Compound 6 did not contain any hydrogen bond forming sites and did not form any gels. Compound 7 formed seven gels, mostly in alkanes. Because the gels were formed in non-hydrogen bonding solvents, the formation of the gels was suggested to be based on hydrogen bonding between the gelator molecules rather than between the gelator and the solvent. This was confirmed by FTIR together with temperature- and concentration-depended 1H NMR-measurements. Compound 8, containing the largest number of hydrogen bond-forming sites within the series, formed only two gels. Too strong interactions between the molecules were speculated to hinder the gel formation.25b
An oligomeric compound 9 consisting in average of three cholesterols and three chains with ferrocene moieties (Fig. 7) formed a film on top of a water solution by solution casting method.25c The permeability studies for salts and water soluble colorants revealed that the films did not permeate red dye or copper salt (Fig. 8). Furthermore, the film was observed to support a steel ball weighing 0.88 grams, indicating better physical and chemical endurance compared to other films formed by some previously reported ferrocene-cholesterol conjugates.
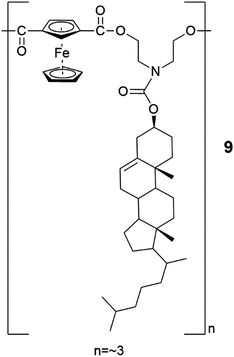 | ||
| Fig. 7 An oligomer comprising of ferrocene and cholesterol (compound 9).25c | ||
2.2 Titanocene derivatives
Titanocene differs from ferrocene not only with respect of the central atom, but also on the additional two chlorines around the metal centre26 leading to a distorted tetrahedral shape instead of the sandwich structure, which is typical for ferrocene. Titanocenes are Lewis acids, which enhances their ability to self-assemble in both polar and non-polar solvents. Titanocene derivatives containing a steroidal entity belong to the group of ALS-compounds.24The titanocene moiety was found to be a prerequisite for gel formation in the series of cholesterol derivatives of titanocene (Fig. 9, compounds 10–16).24,27 Moreover, the length and the substituents of the carbon chain were reported to effect on gelation abilities of compounds 10–16. The fully substituted titanocene in compound 16 was speculated to enhance the solubility of the compound to an extent capable of inhibiting gelation.24,27 When comparing the gelation properties of compounds 10, 12, 14, and 15, however, monosubstitution on the titanocene was found to have less impact on the gelation tendency than the length or substitution of the carbon chain. Consequently, the gel forming abilities of compounds 14 and 15 bearing monosubstituted titanocene entities were similar to the gelation behaviour of compound 10 with a non-substituted titanocene moiety. The gel forming tendency of compound 12 with a non-substituted titanocene but a substituted carbon chain, on the contrary, was less effective when compared with compounds 14 and 15. This was hypothesized to be due to the bulky cyclohexylidene groups. The lack of substituents on the carbon chain was interestingly observed to scale the gelation ability down as compound 13 did not form any gels. A longer carbon chain, on the other hand, had an adverse effect on the gel formation ability, which was obvious from the comparison between compounds 10 and 11. The addition of one methylene group to the chain had significant effects on the gel formation, which was speculated to be due to a more flexible structure.
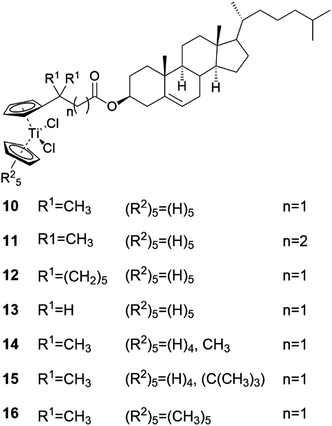 | ||
| Fig. 9 Cholesteryl titanocenyl derivatives 10–16.24,27 | ||
The impact of the substituents on the carbon chain was studied in more detail by comparing the conformations and gelation tendencies of compounds 10, 12, and 13.27 The supergelator property of compound 10 was speculated to stem from multiple favourable conformations arising from methyl groups and hydrogens of similar size. In the case of compound 13 with an unsubstituted carbon chain, the ester group between the steroid and the carbon chain was found to point away from the titanocene part, resulting in incapability of gel formation. On the contrary, compound 12 with the geminal cyclohexylidene groups as substituents got the ester group to orient towards the titanocene moiety resulting in gel formation in both polar and non-polar solvents. Although compound 12 formed gels in higher concentrations than compound 10, the orientation of the ester group clearly had an impact on the gel formation properties.
Gels formed by compound 10 were found to be thermoreversible during temperature-depended 1H NMR and CD measurements.24 With both methods the self-assembly of the gel network and the structural chirality of the gel induced by the cholesterol moieties were observed. In TEM-, cryo-SEM-, and AFM-images both left- and right-handed rope-like bundles of fibres were detected. This handedness of fibres was probably due to the structure resembling spiral staircase brought about by cholesterol moieties (Fig. 10).
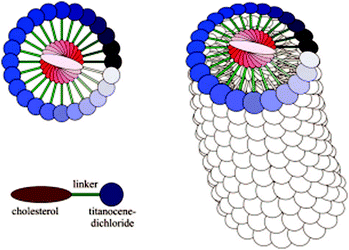 | ||
| Fig. 10 Cholesterol staircase. Reprinted with permission from A. Gansäuer et al., Organometallics, 2009, 28, 1377. Copyright 2009 American Chemical Society. | ||
From the TEM- and cryo-SEM-images of the gels formed by compounds 10, 11, and 12 rope-like bundles of fibres were detcted.27 Tightness of the assemblies varied from compound 10 forming the tightest and compound 11 the loosest assemblies. Moreover, compound 10 formed 3D-networks, where single fibres were connecting the rope-like assemblies together. These connecting fibres were, however, not present in the xerogels obtained from compound 12, implicating steric hindrance in the self-assembly process due to the bulky substituents in the carbon chain. In the gel formed by compound 10 both left- and right-handed assemblies were observed in the TEM- and AFM-images. Based on the CD experiments, the left-handed assemblies were observed to dominate over the right-handed ones. Temperature-depended CD revealed the self-assembly to be non-racemic and induced by van der Waals-forces between the cholesterol moieties.
2.3 Metallogels formed by bile salts
The lanthanide ions are extensively studied owing to their exceptional luminescence properties.28 The colour of the emission is sharp and characteristic to each element in the lanthanide series. Additionally, lanthanide emissions have long lifetimes. To achieve an intense emission, the lanthanide has to form a complex with a suitable ligand. When lanthanides are combined with bile salts and a suitable solvent, the resulting materials have shown potential as emitting coating agents with chromophores,15a,b as templates for nanomaterials16c,d and nanostructures,17,29 and as new composite materials.30 They have even shown enzyme-induced luminescence20b,c,31 opening up possibilities for medical applications.The research group of Maitra15 has studied extensively the use of lanthanide ions in bile salt-derived supramolecular gel systems. As an example, pyrene chromophore was introduced inside the sodium cholate (Fig. 11, compound 17) micelles to which Eu(III)–salt solution was added with the aim of obtaining a more intense lanthanide emission.15a As a result a metallogel, in which the chromophores were in close vicinity of the lanthanide ions, was formed. The formed gels were opaque, which made comparison between the fluorescence spectra of the gels with and without the chromophore feasible. Gels with the chromophore had almost 1000% more intense luminescence compared with the gel lacking the chromophore. In addition to Eu(III), sodium cholate was found to form hydrogels with several other lanthanides including Nd(III), Sm(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III), and Yb(III).15b Rheological studies indicated a decrease in the elasticity of the gel as a function of the size of the lanthanide in question. The most elastic gel was formed with Nd(III) and the least elastic one with Yb(III).
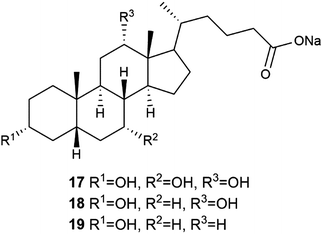 | ||
| Fig. 11 Sodium salts of the bile acids.37 | ||
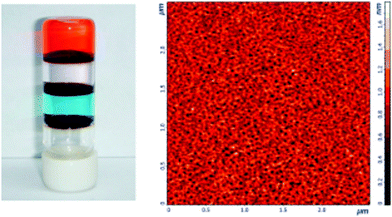
With the aim of making the Tb(III)-cholate hydrogel sensitive to UV-light, the effect of different chromophores were studied.15b Of the tested chromophores, 2,3-dihydroxy naphthalene (DHN) proved to be the best one. By preparing a series of cholate gels containing Tb(III) and Eu(III) in different ratios with DHN, divergent colours under UV-light were observed (Fig. 12). Interestingly, the emissions of the lanthanide cholate xerogels were more intense than the emissions of the native gels despite of the less efficient energy transfer from the chromophore to Yb(III) or Eu(III) in the dry gel.
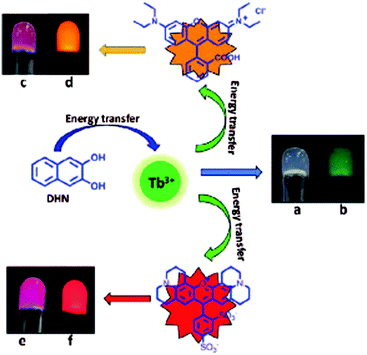
Tb(III)–cholate hydrogel with DHN as a sensitizer (Fig. 13a and b) was further utilized as a scaffold for luminescence resonance energy transfer (LRET).13c Hybrid gels with organic dye (rhodamine B or sulforhodamine 101) as an additional component experienced a cascade energy transfer based on time delayed excitation spectra (Fig. 13c–f). Energy transfer occurred from DHN to Tb(III) and finally to the dye. The concentration of the sensitizer (DHN) was observed to effect on the Tb(III) emissions as well as on the emissions of the dyes. With higher DHN concentration the emission intensities were increased. Xerogels were also found to be luminescent indicating that drying of the gel did not effect on the morphology of the gel. In the self-assembly model proposed, Tb(III) was speculated to act as a directing agent for gel fiber formation for the cholate molecules.13c The DHN molecules and the dyes then attach on the gel fibres by hydrophobic interactions. As DHN chelates Tb(III), energy transfer from Tb(III) to the dye is enabled. By confocal microscopy it was actually confirmed that the dye molecules favour attachment to the gel fiber.
The electron rich and aromatic DHN was utilized as a chromophore also in gels derived from sodium deoxycholate (Fig. 11, compound 18) and Tb(III).15c A series of five different electron deficient aromatic nitro compounds was added to the Tb(III)-deoxycholate gel in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Interestingly, 2,4,7-trinitrofluorenone (TNF) caused the emission to decrease by 63%. This phenomenon may have use in mining industry for detection of nitro compounds.
1. Interestingly, 2,4,7-trinitrofluorenone (TNF) caused the emission to decrease by 63%. This phenomenon may have use in mining industry for detection of nitro compounds.
In a recent study by Laishram and Maitra15d the role of DHN in Eu(III)-cholate gels in different solvents was investigated. Due to quenching of Eu(III) luminescence by water it was expected that the Eu(III) luminescence, sensitized by DHN, in a Eu(III)-cholate hydrogel would be weaker than in a Eu(III)-cholate gel formed in methanol. Surprisingly, no Eu(III) emission was detected in the organogel under UV light. Based on extensive spectrometric and quantum yield measurements, it was concluded that in the case of the methanol gel the DHN is primarily in the bulk solvent. Consequently, the DHN molecules and the Eu(III) ions are too far for the energy transfer. In the hydrogel, on the contrary, the DHN molecules are in hydrophobic pockets formed by the gel fibres, which allows energy transfer and thus the Eu(III) emission can be observed.
Qiao and coworkers32 have been active in the field of lanthanide-containing bile salt-derived supramolecular gels as well. In their study involving Eu(III)-cholate hydrogels,32 the Eu(III)-ions were embedded inside nanofibers formed by cholate molecules. Because the Eu(III) ions were protected against the luminescence quenching by water, enhanced Eu(III) emission was observed. When the cholate concentration was increased, the emission and mechanical stability of the gel were enhanced until a point, where excess of the cholate resulted in a breakdown of the hydrogel, was reached. On the other hand, two divergent emissions were detected at low cholate concentrations, suggesting that there are two distinct environments for the Eu(III). The Eu(III)-cholate hydrogels were utilized in the preparation of Eu2O2S and Eu(III) doped Gd2O2S and Y2O2S nanotubes.
Sodium cholate (Fig. 11, compound 17) and lanthanum chloride formed a gel in water at concentrations of as low as 0.04 wt%.11 Based on DSC and rheology, heating of the gels promoted gelation and enhanced the stiffness of the gels. In TEM, SEM, and FESEM measurements temperature-depended morphology changes were observed (Fig. 14). At 4 °C hollow nanotubes were detected, but when the sample was incubated at 15 °C the number of nanotubes decreased and right-handed nanohelices started to prevail. Intertwined nanoribbons and right-handed coiled-coil rope- and pseudorope-like structures were also observed at 15 °C. At room temperature right-handed twisted and untwisted nanoribbons were present, whereas at 50 °C untwisted nanoribbons prevailed. The size of the structures increased as a function of the temperature.
Wang et al. have contributed to the study of lanthanide-based bile salt-derived metallogels by investigating the microstructures, rheological properties, and fluorescence of hydrogels formed with different ratios of sodium deoxycholate (Fig. 11, compound 18) and Eu(NO3)3.33 With equimolar amounts of 18 and Eu(III) the system self-assembled into homogeneous hydrogel at room temperature exhibiting a red-light emission under UV irradiation. With continuous addition of the europium salt a transition from a gel to a two-phase system took place. The intensity of the photoluminescence of the hydrogel was observed to strongly depend on the sodium deoxycholate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu(III) ratio having the maximum emission at the molar ratio of 3
Eu(III) ratio having the maximum emission at the molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Based on FTIR it was deduced that the lanthanide-deoxycholate coordination was bidentate in nature.
1. Based on FTIR it was deduced that the lanthanide-deoxycholate coordination was bidentate in nature.
Bile salts have reported to form gels with other metal ions as well. For example sodium cholate formed gels in water with a series of metal ions (Fig. 15).17 Except of Ca(II)-cholate, which required gentle heating, the gels were formed at room temperature. pH was observed to play a crucial role in the gelation process. Upon addition of the Lewis acidic metal ions, cholate anions were formed from the sodium cholate. This affected on the hydrogen bonding environment of the molecules leading either to aggregation or gelation.
 | ||
| Fig. 15 Metal-cholate metallogels formed in water. Reproduced from A. Chakrabarty et al., J. Mater. Chem., 2012, 22, 18268, with permission from the Royal Society of Chemistry. | ||
The morphologies of the gels were different depending on the metal. Cd(II)- and Zn(II)-containing gels possessed tubular and entangled fibres (Fig. 16a and b) whereas the Co(II)-containing gel consisted of intertwined fibres experiencing some twisting (Fig. 16c). Very thin fibers forming a porous mesh was observed in the case of the Cu(II)-containing gel (Fig. 16d). Ca(II)-cholate gel exhibited different morphologies with regard of the counter anion. The chloride salt induced formation of predominantly flat tubes, whereas with the nitrate salt nanohelices were observed. The divergent morphologies were speculated to stem from the delicate variations in the pH related to the counter anion.
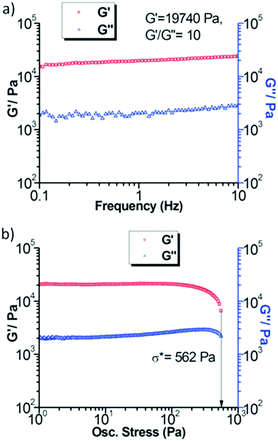
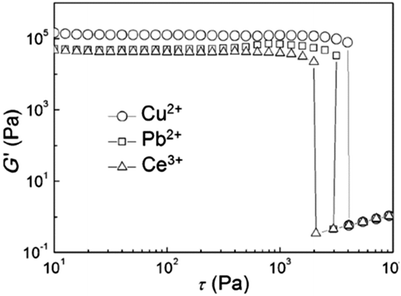
Dynamic rheology measurements for the Co(II)-cholate hydrogel (Fig. 17) revealed that the gel is mechanically very stable. This was obvious from the linear domain of deformation in the frequency sweep test combined with the high yield stress value in the stress sweep test. In both of the tests the G′ (elasticity) was larger than G′′ (stiffness), indicating that the Co(II)-cholate hydrogel is a viscoelastic soft solid material.
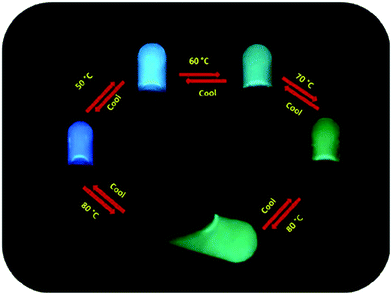
Transparent Ag(I)-cholate gel consisted of spherical aggregates of various sizes, which formed a network bonding loosely to each other.34 The gel was thermoreversible and experienced shear thinning. In addition, the gel formed again after shaking or applying pressure without any loss of mechanical strength. Based on the fluorescence lifetime measurements with pyrene, prodan (6-propionyl-2-dimethylamino naphthalene), and DPH (1,6-diphenylhexatriene) the cholate molecules were deduced to be compactly packed. Moreover, the gel was detected to contain areas with high hydrophobicity. The Ag(I)-cholate gel with prodan exhibited divergent colours at different temperatures (Fig. 18). Such colour changes were not observed, when a Na(I)-cholate gel containing prodan was heated.
Indium(III) cholate and deoxycholate formed gels only after sonication of the samples.29a The formation of the deoxycholate gels was much slower than the formation of the cholate gels. Gelation was not observed with Al(III) and Ga(III). Elemental analysis, ESI-MS, and FT-IR measurements indicated that the gels consisted of In(III) and cholate/deoxycholate in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, where the cholate chelates In(III) in bidentate fashion. When the In(III)-cholate/deoxycholate gels were studies by SEM and AFM both before and after the gel formation, divergent structures were observed. In samples taken immediately after sonication but before the formation of the gel, spherical aggregates were detected. On the contrary, in the samples taken 1 h after sonication, which induced the gel formation, entangled fibres were seen. The gelation was suggested to begin by the formation of the 1
3 ratio, where the cholate chelates In(III) in bidentate fashion. When the In(III)-cholate/deoxycholate gels were studies by SEM and AFM both before and after the gel formation, divergent structures were observed. In samples taken immediately after sonication but before the formation of the gel, spherical aggregates were detected. On the contrary, in the samples taken 1 h after sonication, which induced the gel formation, entangled fibres were seen. The gelation was suggested to begin by the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 In(III)
3 In(III)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholate complexes. After that the complexes were speculated to self-assemble via hydrogen bonding and hydrophobic interactions between the cholate moieties into larger aggregates, which further self-assemble into a nanofibrous network responsible for the gel formation.
cholate complexes. After that the complexes were speculated to self-assemble via hydrogen bonding and hydrophobic interactions between the cholate moieties into larger aggregates, which further self-assemble into a nanofibrous network responsible for the gel formation.
Lithocholate (Fig. 11, compound 19) was found to form hydrogels with alkali cations (Li(I), Na(I), K(I), Rb(I), and Cs(I)).16b Similar to the La(III)-cholate hydrogels reported previously, the mechanical strength and the size of the structural elements increased as a function of the temperature also in the case of these alkali metal-lithocholate hydrogels.32 Furthermore, the size of the cation affected on the stability and the transparency of the gel. The gel formed with Li(I) was the most stable and opaque, whereas the gel formed with Cs(I) was the weakest and more transparent.16b The different appearances of the gels was observed to stem from divergent morphologies detected by TEM and cryo-TEM measurements. Gels with Li(I) possessed nanotubules with divergent sizes, whereas the nanotubules in the cases of gels with Na(I), K(I), and Cs(I) were consistent in sizes. The increased mechanical strength observed with the increasing temperature was accompanied with a change in the appearance of the gel from transparent to opaque. Rb(I)-lithocholate gels required the highest temperatures to change, whereas Cs(I) gels disassembled when the temperature was increased. These alkali metal-lithocholate hydrogels were able to absorb virtually completely positively charged dyes (methylene blue and rhodamine 6G) in 20 minutes (Fig. 19). This phenomenon can be potentially utilized in the wastewater purification.
Divalent and trivalent metal ions were observed to induce metallogel formation of sodium lithocholate (Fig. 11, compound 19) in water.16a It was shown that gels formed with different metal ions experienced similar mechanical stabilities (Fig. 20). The Cu(II)-lithocholate hydrogels were selected to further studies. TEM revealed that the gel consisted of a 3D fiber network showing remarkable mechanical stability that was hardly influenced by the metal ion concentration. Furthermore, the gels were thermally stable retaining mechanical strength even when heated to 90 °C. The Cu(II) hydrogel also experienced pH responsiveness. The viscosity of the solution increased as the pH was decreased from alkaline to neutral, where a gel was finally formed. Based on the FTIR spectra, the dominant interaction in the Cu(II) hydrogel was the intermolecular hydrogen bonding between the cholate molecules combined with a unidentate coordination to Cu(II) ions.
Cationic and neutral bile acid derivatives formed gels in water as well as in aqueous metal salt solutions of NaCl, NaNO3, KCl, LiCl, and BaCl2, for example.35 For some derivatives gel formation required the presence of the metal. In the case of cationic derivatives the deoxycholic acid-derived ones were the best gelators. This is surprising, since deoxycholic acid derivatives do not have the tendency to form gels, as numerous studies have shown before.36
Metallogel formation of different bile salts (Fig. 11 and 21) with various metal ions in water with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (bile acid salt
1 (bile acid salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
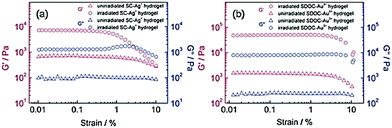
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ion) molar ratios was observed by Shen et al.37 Sodium taurocholate (compound 22) did not form any gels with the tested metal ions, whereas other bile acid salts assembled into gels with all, or some of, the metal ions tested. Sodium lithocholate (compound 19), for example, formed gels with all the metal ions tested (Fig. 22), whereas sodium glycocholate (compound 21) only formed five metallogels. The hydrogels formed with Ag(I) and Au(III) formed at specific pH-ranges depending on the bile salt that was used. Based on rheology (Fig. 23), the mechanical strength of the Ag(I) and Au(III) hydrogels of compounds 17 and 18 increased when the gels were irradiated with natural light in controllable light incubator (800 lux at 23 °C). This was speculated to stem from the formation of Ag- and Au-nanoparticles by photoreduction. The particles could then promote linking of the gel fibres together. However, based on the FESEM images, the irradiated and non-irradiated gels had similar morphologies consisting of 3D networks of cross-linked fibres.
metal ion) molar ratios was observed by Shen et al.37 Sodium taurocholate (compound 22) did not form any gels with the tested metal ions, whereas other bile acid salts assembled into gels with all, or some of, the metal ions tested. Sodium lithocholate (compound 19), for example, formed gels with all the metal ions tested (Fig. 22), whereas sodium glycocholate (compound 21) only formed five metallogels. The hydrogels formed with Ag(I) and Au(III) formed at specific pH-ranges depending on the bile salt that was used. Based on rheology (Fig. 23), the mechanical strength of the Ag(I) and Au(III) hydrogels of compounds 17 and 18 increased when the gels were irradiated with natural light in controllable light incubator (800 lux at 23 °C). This was speculated to stem from the formation of Ag- and Au-nanoparticles by photoreduction. The particles could then promote linking of the gel fibres together. However, based on the FESEM images, the irradiated and non-irradiated gels had similar morphologies consisting of 3D networks of cross-linked fibres.
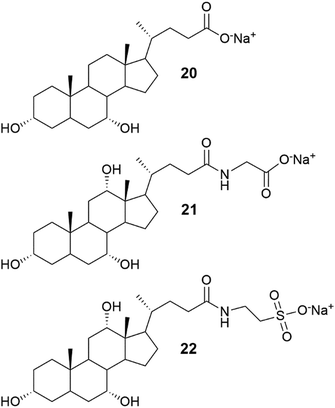 | ||
| Fig. 21 Structures of sodium chenodeoxycholate (20), sodium glycocholate (21), and sodium taurocholate (22).37 | ||
 | ||
| Fig. 22 Hydrogels formed by compound 19 with various metal salts. Reproduced from J.-S. Shen et al., Soft Matter, 2013, 9, 2017, with permission from the Royal Society of Chemistry. | ||
Bile salt-derived metallogels have also been used in generating hybrid materials exhibiting beneficial properties. Examples include the use of Ca-doped Cd-cholate hydrogel in the presence of Na2SeSO3 as a template for synthesizing yellow-emitting CdSe quantum dots at room temperature30b and enhancement of the emission and stability of blue emitting nano clusters by incorporating them into a zinc-cholate hydrogel.38
The hybrid materials involving steroidal metallogels may find great potential in designing optoelectronic devices, in photocatalysis, or in biomedical and sensor applications.
2.4 Heteroatom-containing systems as coordination environments
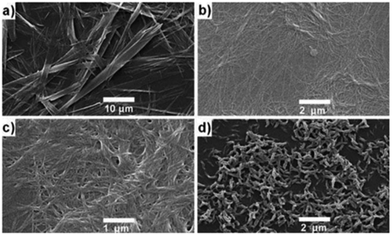
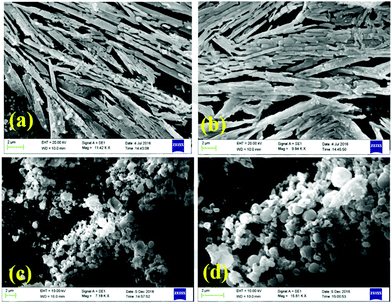
Nitrogen and sulphur heterocycles and crown ethers are intriguing molecules regarding self-assembly with metal ions, because of their ability to form hydrogen bonds.10,13a,b,14 Coordination to metal ions may change the properties of the heterocycle (or crown ether) leading to possibilities of developing new applications in the fields of molecular recognition, medicine, and catalysis.Two steroid derivatives with attached nitrogen heterocycles capable of binding platinum (Fig. 24) were found to slowly form gels in various solvents.39 Gels that were formed in solvent mixtures were more stable than gels that were formed in a single solvent. Compound 23 was observed to induce gelation in fewer occasions, but gels were more thermostable than gels formed by compound 24. SEM-images of xerogels revealed divergent morphologies of the gel structures between the two compounds as well as between gels formed in different solvents (Fig. 25). In PhCN and DMA compound 24 formed long, thin fibres assembling into a dense network providing evidence for the better gel forming ability of the compound. Gels formed by compound 23 in the same solvents consisted of wide, ribbon-like fibres. Addition of EtOH or MeOH to DCE or chloroform affected on the morphology of the gels formed by compound 24 producing short, thick, and twisted fibres. Addition of hexane or acetone to DCE, on the other hand, revealed the gel fibres to resemble long and thin wool fibres.
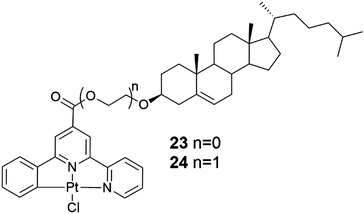 | ||
| Fig. 24 Two steroid derivatives with attached nitrogen heterocycles capable of binding platinum.39 | ||
The chiral recognition abilities of the gels were also studied.39 Addition of (S)- or (R)-BINAP with heating to the 1% metallogel (compound 23 in chloroform and compound 24 in toluene) resulted in the collapse of the gel structure. This was suggested to originate from the bulky BINAP replacing the chlorine coordinated to platinum resulting in the inability of the heteroaromatic moieties to interact with one another. The gel network formed by compound 23 in chloroform collapsed after adding only 0.1 equivalents of (R)-BINAP to the chloroform gel making it more sensitive in chiral recognition compared to compound 24. The addition of (R)-BINAP to the gel gave rise to larger, denser, and longer crystalline rods compared to the addition of (S)-BINAP, respectively. Addition of other phosphine ligands similarly caused collapse of the gel.
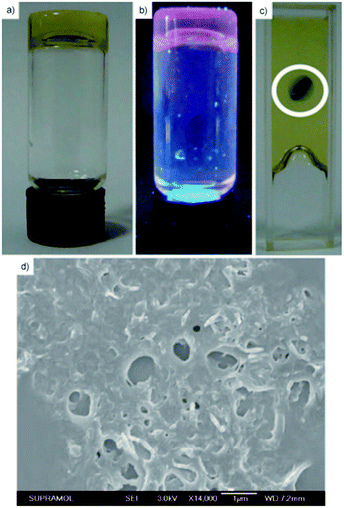
The cholesterol derivative of platinum bipyridine (Fig. 26, compound 25) self-assembled into gels at room temperature in aliphatic solvents, such as octane.40 It formed an exceptionally strong gel in dodecane, which was stable even after heating up to 117 °C, a temperature near the decomposition temperature of the platinum complex. SEM images showed a 3D-network formed by cross-linked fibres (Fig. 27d). Interestingly, a prolonged exposure of the gel to UV-light at 15 °C induced a colour change from yellow to blue (Fig. 27a–c). Blue colour faded back to yellow after the exposure.
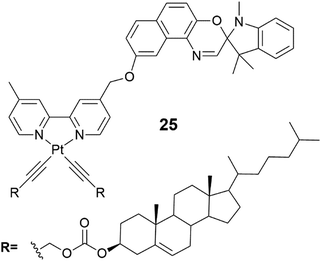 | ||
| Fig. 26 Compound 25.40 | ||
The gelation properties of cholesterol based terpyridyl platinum derivatives 26 and 27 (Fig. 28) depended on the substitution of the terpyridyl moiety.41 Compound 27 formed two gels at high concentrations (25 mg mL−1) being nearly insoluble in most organic solvents. Compound 26 with tert-butyl substituents, for one, was soluble in most polar solvents, but formed three gels in different concentrations in toluene, ethyl acetate, and n-propanol. If the samples used for testing the gelation tendencies were not treated with ultrasound after heating, compound 26 precipitated out from the solution. The red precipitate formed in n-propanol did not experience a colour change after heating. However, the gels formed in n-propanol and toluene changed colour: the liquid sample was red and after the ultrasound treatment the gel changed from opaque to yellow.
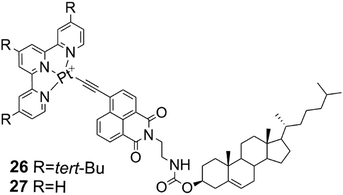 | ||
| Fig. 28 Compounds 26 and 27.41 | ||
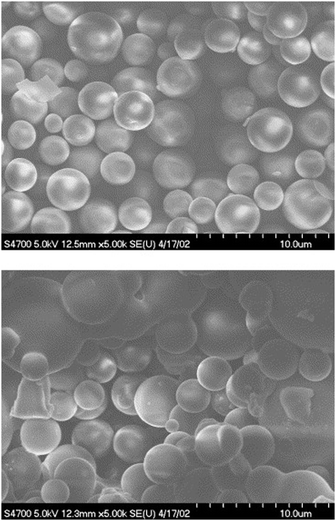
The SEM- and TEM-images of the xerogel of compound 26 in n-propanol showed that the gel network comprised of ribbon-like, slightly twisted fibres.41 Based on X-ray crystallography and surface wettability testing the structure of the gel was observed to form of layers. The layers were comprised of ribbon-like structures with hydrophobic cholesterol on the outside (Fig. 29, below). The precipitate, on the other hand, was detected to consist of spherical structures, which were revealed to be vesicles by TEM. The vesicle wall was found to consist of a single molecular layer with the cholesterol parts inside the vesicle (Fig. 29, above). The acetonitrile gel of 27, for one, possessed a wrinkled surface structure covered with fibres, whereas the precipitate was observed to consist of knot-like structures with wrinkled surface.
Compounds 28 and 29 (Fig. 30) spontaneously assembled into gels during chromatography and NMR-, MS-, and IR-measurements.42 Compound 28 acted as a supergelator, whereas compound 29 formed gels in higher concentrations (as 1%). During storage at room temperature the gels formed by compound 29 were observed to turn into solutions but were formed again after heating. When irradiated with laser beams gels of both 28 and 29 experienced Tyndall effect. Based on SEM-images the gel network comprised of compound 28 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O
1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH consisted of grass-like fibres, whereas compound 29 was found to form ball-like structures both in solution and in the gel state (Fig. 31). The spherical morphology was suggested to stem from the steric hindrance of Zn-coordinated phenantroline, preventing the formation of fiber-like structures.
MeOH consisted of grass-like fibres, whereas compound 29 was found to form ball-like structures both in solution and in the gel state (Fig. 31). The spherical morphology was suggested to stem from the steric hindrance of Zn-coordinated phenantroline, preventing the formation of fiber-like structures.
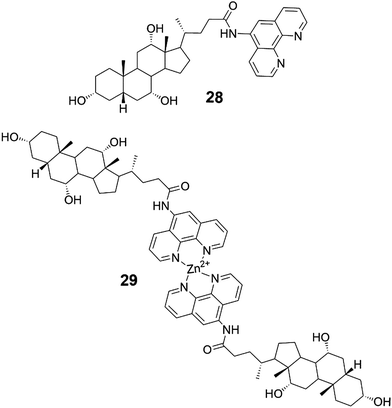 | ||
| Fig. 30 The conjugate of cholic acid and phenantroline 28 and its dimeric Zn-complex 29.42 | ||
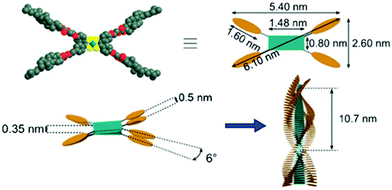
From the three pyridine derivatives of cholesterol (Fig. 32) only compound 31 was observed to form a metallogel with Ag(I)OTf in diphenyl ether, despite of the better gel forming abilities of compounds 30 and 32 in pure solvents.43 The perpendicular orientation of the pyridyl nitrogen with respect of the cholesterol moiety and the positions of the adjacent pyridyl nitrogens (appr. 180°) in the helical staircase assembly (see Fig. 10) were speculated to promote the metallogel formation.
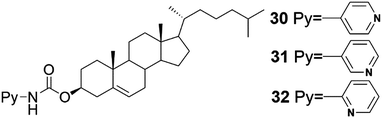 | ||
| Fig. 32 Compounds 30–32.43 | ||
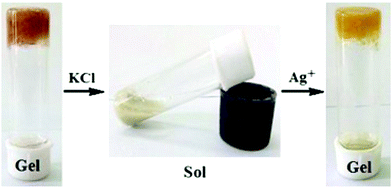
The benzimidazole derivative of cholesterol (Fig. 33, compound 33) was observed to form brown metallogels with silver(I) ions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) DMF
1 (v/v) DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O.9j Without the silver salt 33 formed a partial gel in 1
H2O.9j Without the silver salt 33 formed a partial gel in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) DMF
1 (v/v) DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and no gels in other solvents or with other metal salts (Fig. 34). Based on the FTIR and 1H NMR measurements combined with UV-vis and fluorescence titrations, the nitrogen on the benzimidazole moiety was responsible for the metal-coordination and also together with the ester group for the self-assembly leading to gelation. The partial gel without the silver salt had a different morphology compared to the silver gel (Fig. 35). The metallogel consisted of plate-like assemblies stacked on top of each other, whereas the partial gel contained spherical assemblies.
H2O and no gels in other solvents or with other metal salts (Fig. 34). Based on the FTIR and 1H NMR measurements combined with UV-vis and fluorescence titrations, the nitrogen on the benzimidazole moiety was responsible for the metal-coordination and also together with the ester group for the self-assembly leading to gelation. The partial gel without the silver salt had a different morphology compared to the silver gel (Fig. 35). The metallogel consisted of plate-like assemblies stacked on top of each other, whereas the partial gel contained spherical assemblies.
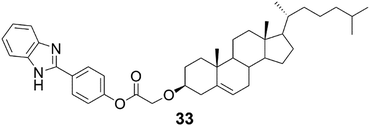 | ||
| Fig. 33 The benzimidazole derivative of cholesterol (compound 33).9j | ||
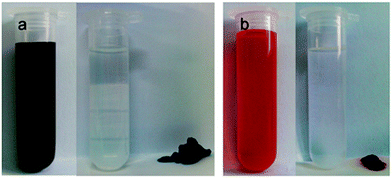
The silver gel of compound 33 was not thermoreversible and collapsed in the presence of Cl− ions as AgCl formed and precipitated out from the gel network.9j However, the gel formed again after adding a sufficient amount of Ag(I). Interestingly, the colour of the gel was lighter than before the addition of the Cl− ions (Fig. 36). Impedance spectroscopy in various temperatures revealed that the metallogel was able to conduct ions when thermally triggered. Rheology and impedance spectroscopy explained the ability by the presence of a disorganized rubbery state within the silver gel.
Cholesterol appended diazine derivative (Fig. 37, compound 34) on a 4-hydroxybenzaldehyde scaffold formed gels with Ag(I) and Fe(III) ions in CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH (3
CH3OH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).44 With the aid of FTIR and 1H NMR the authors suggested a gelation model, where the diazine group coordinates the metal ions, which assist in the formation of the gelator network. The strong hydrophobic interactions of the cholesteryl moieties further reinforce the aggregation. When tetrabutylammoniun chloride was added to the Ag(I)-gel of 34 it broke down completely, whereas under identical conditions the Fe(III)-gel remained undisturbed. All the other halides (TBAF, TBABr, and TBAI), however, were unable to induce similar gel–sol transformation of the Ag(I)-gel. When TBAI was added into the Fe(III)-gel, a colour change from orange-red to orange, but no phase change, was observed. These properties demonstrate the usefulness of these metallogels in visual recognition and discrimination of halides. The Ag(I)-gel of 34 remained intact upon addition of NH4SCN, whereas the Fe(III)-induced gel turned into a solution meaning that also the gels with different metal ions can be visually distinguished. It was also observed that the Fe(II) ions did not distract the Fe(III)-promoted gelation. This is very promising, since most of the Fe(III)-sensing probes suffer from interference by the Fe(II) ions. In addition to acting as materials for visual discrimination of halides or as probes for Fe(III)-sensing, the Ag(I)- and Fe(III)-metallogels of 34 were able to effectively adsorb uranine and picric acid from water being recyclable in the process.
1, v/v).44 With the aid of FTIR and 1H NMR the authors suggested a gelation model, where the diazine group coordinates the metal ions, which assist in the formation of the gelator network. The strong hydrophobic interactions of the cholesteryl moieties further reinforce the aggregation. When tetrabutylammoniun chloride was added to the Ag(I)-gel of 34 it broke down completely, whereas under identical conditions the Fe(III)-gel remained undisturbed. All the other halides (TBAF, TBABr, and TBAI), however, were unable to induce similar gel–sol transformation of the Ag(I)-gel. When TBAI was added into the Fe(III)-gel, a colour change from orange-red to orange, but no phase change, was observed. These properties demonstrate the usefulness of these metallogels in visual recognition and discrimination of halides. The Ag(I)-gel of 34 remained intact upon addition of NH4SCN, whereas the Fe(III)-induced gel turned into a solution meaning that also the gels with different metal ions can be visually distinguished. It was also observed that the Fe(II) ions did not distract the Fe(III)-promoted gelation. This is very promising, since most of the Fe(III)-sensing probes suffer from interference by the Fe(II) ions. In addition to acting as materials for visual discrimination of halides or as probes for Fe(III)-sensing, the Ag(I)- and Fe(III)-metallogels of 34 were able to effectively adsorb uranine and picric acid from water being recyclable in the process.
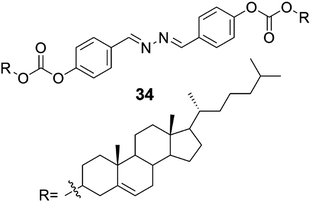 | ||
| Fig. 37 The structure of compound 34 capable of forming organogels with Ag(I) and Fe(III) ions.44 | ||
Organogels formed by cholesterol azopyridine conjugate (Fig. 38, compound 35) exhibited metal ion-mediated helicity inversion through metal ion coordination.45 Compound 35 was able to form self-supporting gels after addition of Ni(II), Cu(II), Mn(II), Mg(II), Fe(II), Bi(III), and Eu(III). The gels were studied by microscopic techniques combined with FTIR, 1H NMR, and CD measurements. The 1H NMR experiments strongly indicated that the metal ions were coordinated with the pyridyl unit rather than with the azo moiety. When changing the metal ion from Ni(II) to Cu(II), the M-helical structure and negative CD peak were transformed into P-helical nanoribbons and positive CD signals indicating that metal ions were able to induce chirality difference in the gels. Based on CD and SEM it was established that P-type helical aggregates showed positive Cotton effects at 400–500 nm, while M-helical aggregates exhibited negative Cotton effects at the same region. The chirality of the gels depended not only on the metal ion but also on the solvent matrix. For example, the Ni(II)-gel showed M-helicity in n-butanol but nanostructures with P-helix were obtained in p-xylene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH. It was observed that in solvents with relatively low polarity most of the complexes displayed P-chirality. In addition to the fascinating properties of the metallogels of 35, the gel formed by the compound itself was prone to E to Z photoisomerization upon UV light irradiation.
EtOH. It was observed that in solvents with relatively low polarity most of the complexes displayed P-chirality. In addition to the fascinating properties of the metallogels of 35, the gel formed by the compound itself was prone to E to Z photoisomerization upon UV light irradiation.
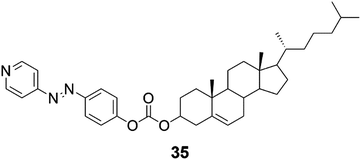 | ||
| Fig. 38 The azo-cholesterol conjugate 35 capable of exhibiting phototriggered dimensional transition and controllable supramolecular chirality.45 | ||
Cholesterol derivatives NiC2 and NiC6 containing bis(dithiolene) moiety (Fig. 39, compounds 36 and 37) were found to form gels in linear alkanes (C7–C12).46 The transparent, thermoreversible, and robust gels were stable for several months. NiC2 was observed to form gels with lower concentrations than NiC6. In dodecane NiC2 was found to commence gelation as low a concentration as to be categorized as a supergelator. On the contrary, NiC11 (Fig. 39, compound 38) and an additionally synthetized NiC0 did not form any gels in linear alkanes, suggesting that the gel formation depended on the length of the carbon chain.
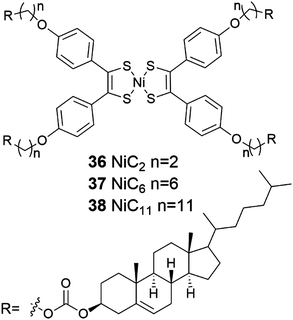 | ||
| Fig. 39 Nickel-bis(dithiolene) complexes conjugated to cholesterol 36–38.46 | ||
Based on SEM and AFM the gel network formed by 36 was consisted of twisted fibers, which were entangled to each other.46 CD measurements revealed that the helicity of the gel fibers was right-handed. The helical fibers were responsible for the chiro-optical effects at the near-infrared region. The same effects, however, were not detected in the solution 36. The formation of the gel was suggested to occur via hierarchical process (Fig. 40), which results in a gel network consisting of entangled twisted fibres formed from 1D helical stacks of the compound in question.
The crown ether–cholesterol conjugate 39 (Fig. 41) was found to form gels in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclohexane
1 cyclohexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzene with divergent melting temperatures depending on the metal ion present.47 The melting temperatures of the gels were observed to increase and then gradually decrease with respect of the increasing concentration of Li(I), Na(I), K(I), Cs(I), Rb(I), and NH4+-salts. Particularly Rb(I) induced an increase in the melting (by 20 °C) when compared with the gel without any metal. Cs(I) had an opposite effect on the melting temperature, and above 15 mol% of Cs(I) the gelation was not even observed. This was suggested to stem from the tendency of the Cs(I)-ions to form 1
benzene with divergent melting temperatures depending on the metal ion present.47 The melting temperatures of the gels were observed to increase and then gradually decrease with respect of the increasing concentration of Li(I), Na(I), K(I), Cs(I), Rb(I), and NH4+-salts. Particularly Rb(I) induced an increase in the melting (by 20 °C) when compared with the gel without any metal. Cs(I) had an opposite effect on the melting temperature, and above 15 mol% of Cs(I) the gelation was not even observed. This was suggested to stem from the tendency of the Cs(I)-ions to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal
2 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) crown sandwich complexes with 18-crown-6, which would then inhibit the gel formation by affecting adversely to the self-assembly process.
crown sandwich complexes with 18-crown-6, which would then inhibit the gel formation by affecting adversely to the self-assembly process.
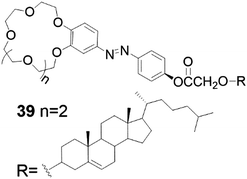 | ||
| Fig. 41 Cholesterol-crown ether derivative 39.47 | ||
2.5 Gels formed by multiple components
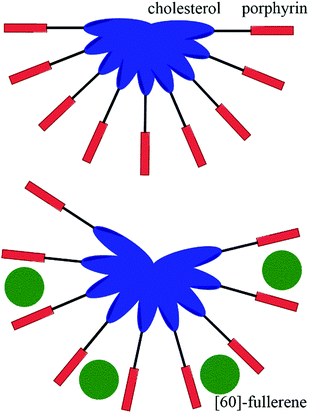
Versatility is the asset of gels formed by multiple components.48 Materials with very distinctive properties can be generated by mixing compounds in different ratios or by changing one or more components. Multiple component gels are commonly prepared by first synthesizing the gelators and then performing gelation tests with varying combinations and ratios of compounds in different solvents. By subcomponent self-assembly time and resources can be saved when screening potential gel forming combinations. Mixing of all the needed components forms the gelating agent in situ, during the gelation test.48Gels formed by compounds 40 and 42 (Fig. 42) were observed to be stronger in the presence of [60]-fullerene.49 For example, gels formed by compound 40 in benzene in the presence of [60]-fullerene were stable up to 20 °C, whereas gels without it decomposed when temperatures exceeded 5 °C. In the case of compound 42 a stable gel was formed only with [60]-fullerene at 5 °C, whereas in the absence of [60]-fullerene gelation was not observed. Compounds 41 and 43 (Fig. 42) did not form any gels in the tested solvents with [60]-fullerene. Based on computational studies the carbon chain connecting the cholesterol and the porphyrin ring tended to bend in the case of compounds 41 and 43 having an odd number of carbons in the carbon chain. In the contrary, compounds 40 and 42 with an even number of carbons in the carbon chain possessed extended conformations. Hence, the extended conformation was more favourable regarding interactions between molecules. The cholesteryl-porphyrins formed a spiral staircase architecture suitable for accommodating the [60]-fullerene molecules between the porphyrin moieties to give rise to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sandwich complexes (Fig. 43), whose structures were evidenced by measuring the melting point of the gel together with UV-vis- and CD-measurements. Based on the IR-measurements interactions between the cholesterol moieties were primarily responsible for the gel formation for compounds 40 and 42, followed by interactions between the porphyrin moieties, and interactions between the porphyrins and [60]-fullerene.
1 sandwich complexes (Fig. 43), whose structures were evidenced by measuring the melting point of the gel together with UV-vis- and CD-measurements. Based on the IR-measurements interactions between the cholesterol moieties were primarily responsible for the gel formation for compounds 40 and 42, followed by interactions between the porphyrin moieties, and interactions between the porphyrins and [60]-fullerene.
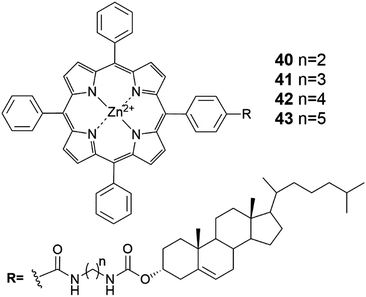 | ||
| Fig. 42 The structures of cholesteryl porphyrins 40–43.49 | ||
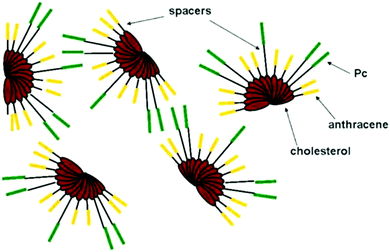
Cholesteryl anthracene (Fig. 44, compound 44) formed thermoreversible and photoactive gels (Fig. 45) in aromatic solvents when mixed with the ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with Zn(II)-phthalocyanine (ZnII-Pc)-containing compounds 45–48.50 Whether the carbon chain connecting the cholesterol and the phthalocyanine moieties possessed an odd or an even number of carbons had no effect on the mechanical stability of the gel contrary to the analogous porphyrin compounds.49 Instead, the gels formed by compounds 45 and 46 with shorter alkyl chains were more stable upon storage compared to gels formed by compounds 47 or 48 with longer alkyl chains.50
1 with Zn(II)-phthalocyanine (ZnII-Pc)-containing compounds 45–48.50 Whether the carbon chain connecting the cholesterol and the phthalocyanine moieties possessed an odd or an even number of carbons had no effect on the mechanical stability of the gel contrary to the analogous porphyrin compounds.49 Instead, the gels formed by compounds 45 and 46 with shorter alkyl chains were more stable upon storage compared to gels formed by compounds 47 or 48 with longer alkyl chains.50
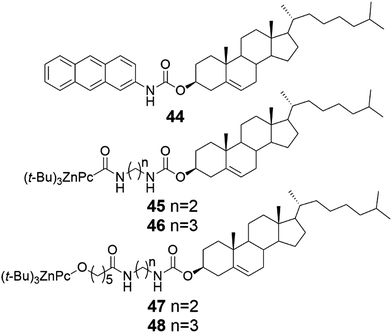 | ||
| Fig. 44 Cholesteryl anthracene 44 and cholesteryl ZnII-Pc-containing derivatives 45–48.50 | ||
Both cholesterol and ZnII-Pc were observed to be necessary for the gel stabilization in the control experiments.50 Compounds 44 and 45–48 were suggested to assemble into fan-like architectures with the cholesterol moieties at the base, enabling π-interactions between the ZnII-Pc-moieties between adjacent assemblies (Fig. 46). Based on the temperature depended UV-vis- and fluorescence measurements the ZnII-Pc-moieties stacked on top of each other. In addition, hydrogen bonding and van der Waals-interactions were found to promote the gel formation based on the temperature-depended FTIR-measurements. The TEM images showed the gel networks to consist of interwoven fibres. CuAAC-reaction in the gels formed by compounds 44 and 45 resulted in higher melting temperature and improved mechanical stability. Moreover, the gels were thermoreversible even after the reaction. TEM images revealed a denser and more entangled gel network after the CuAAC-reaction providing a potential explanation for the higher melting temperature. Based on the FTIR-measurements the gel structure after the reaction was stabilized by hydrogen bonding as well as by van der Waals- and π–π interactions similar to gels before the reaction.
2.6 Stimuli-responsive gels
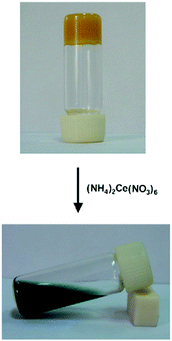
Gels reacting specifically to either physical or chemical stimuli are defined as stimuli-responsive gels.51 Because gels have solid- and liquid-like properties (elasticity and viscosity, respectively) and can react to stimuli in either molecular or gel network level, they are optimal materials in designing responsive materials. Physical stimuli targeting supramolecular gels include mechanical stimuli, such as shaking, as well as light and heat. Potential chemical stimuli inducing responses in supramolecular gel materials may include changes in pH or oxidation levels. If the gel contains dipoles or paramagnetic materials its properties can be affected by electric or magnetic fields. Because of the versatility of potential sources of stimuli, physical gels can be designed to respond to more than one stimulus. This type of gels have potential for various applications in biomedicine and catalysis, for example.Ethyl acetate gel of compound 1, a ferrocene conjugate of cholesterol (Fig. 2), was found to react to addition of an oxidizing agent.12 A solution of the oxidizing agent (NH4)2Ce(NO3)6 in methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with respect to compound 1) was added to the surface of the ethyl acetate gel. The yellow gel turned into a dark green suspension (Fig. 47), which was suggested to stem from the poor solubility of the oxidized form of compound 1 to methanol together with the salt effect. The repulsion between the oxidized ferrocenyl moieties was hypothesized to break the hydrogen bonds between the amide groups leading to the collapse of the gel. After the addition of a reducing agent, SnCl2, the dark green suspension turned yellow indicating reduction of ferrocenyl moieties to ferrocene. However, the gel was not formed even after several heating–cooling cycles. This was most probably caused by the addition of the oxidizing agent in methanol to which compound 1 is insoluble in. Addition of NH4NO3 or Ce(NO3)6 did not result in similar change when added on top of the ethyl acetate gel of compound 1.
1 molar ratio with respect to compound 1) was added to the surface of the ethyl acetate gel. The yellow gel turned into a dark green suspension (Fig. 47), which was suggested to stem from the poor solubility of the oxidized form of compound 1 to methanol together with the salt effect. The repulsion between the oxidized ferrocenyl moieties was hypothesized to break the hydrogen bonds between the amide groups leading to the collapse of the gel. After the addition of a reducing agent, SnCl2, the dark green suspension turned yellow indicating reduction of ferrocenyl moieties to ferrocene. However, the gel was not formed even after several heating–cooling cycles. This was most probably caused by the addition of the oxidizing agent in methanol to which compound 1 is insoluble in. Addition of NH4NO3 or Ce(NO3)6 did not result in similar change when added on top of the ethyl acetate gel of compound 1.
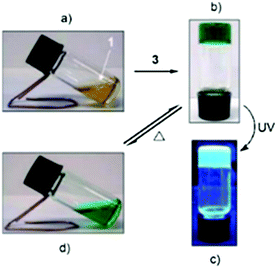
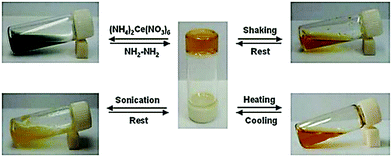
With the help of a film mold, a cyclohexane gel of the supergelator 2 (Fig. 4) formed a gel film, which was stable in room temperature in the wet state and could be bent into a coil-like structure.25a The gel reacted also to four different stimuli, changing from gel to sol to gel again (Fig. 48). When a small amount of water solution containing (NH4)2Ce(NO3)6 (equivalent amount with respect to compound 2) was added on top of the cyclohexane gel, it turned to dark green suspension. After adding hydrazine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with respect to compound 2) with shaking, the orange and transparent gel was re-formed. Similar to the ethyl acetate gel of compound 1 the simultaneous addition of NH4NO3 and Ce(NO3)6 did not disrupt the gel. Vigorous shaking or heating, however, resulted in solution, which restored back as gel upon resting. Sonication produced a viscous suspension, yellower in colour when compared to the original gel. When left undisturbed the suspension returned to a gel.
1 molar ratio with respect to compound 2) with shaking, the orange and transparent gel was re-formed. Similar to the ethyl acetate gel of compound 1 the simultaneous addition of NH4NO3 and Ce(NO3)6 did not disrupt the gel. Vigorous shaking or heating, however, resulted in solution, which restored back as gel upon resting. Sonication produced a viscous suspension, yellower in colour when compared to the original gel. When left undisturbed the suspension returned to a gel.
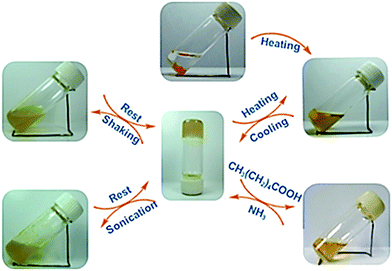
Also a dimeric cholesteryl ferrocene derivative (Fig. 6, compound 7) formed a stimuli-responsive gel.25b In n-decane the thermoreversible gel reformed also after shaking and sonication at room temperature (Fig. 49). Addition of a small amount (<1/200, v/v) of capronic acid, acetic acid, or hydrochloric acid brought about the disruption of the gel. The gel did not re-form after heating and cooling without the addition of ammonia (Fig. 49). This cycle could be repeated several times, during which a precipitate (salt) formed by the acid and ammonia was observed. The collapse of the gel network was speculated to be due to the protonation of the tertiary amine leading to increased repulsion between molecules eventually resulting in the gel turning into a solution.
Interestingly, the ferrocene moiety on 7 was found to be too stable in the gel state to be oxidized by (NH4)Ce(NO3) or Ce(SO4), for example.25b In dichloromethane the colour of the solution turned instantly from yellow to green upon addition of the oxidizer, but in n-decane the change was much slower. Hence it was concluded that the microenvironment of the ferrocene moiety of 7 affected on the rate of the oxidation reaction.
From the cholesterol and estradiol derivatives containing Pt-coordinating moieties (Fig. 50, compounds 49–54), three cholesterol derivatives (compounds 50–52) formed gels in organic solvents.52 Compound 50 formed a gel in decalin at 15 °C and compounds 51 and 52 in cyclohexane at room temperature. Compound 52 formed the most stable gel, which is why it was studied in more detail. The gel was found to re-form after heating and mechanical stress (shaking for 5 minutes).
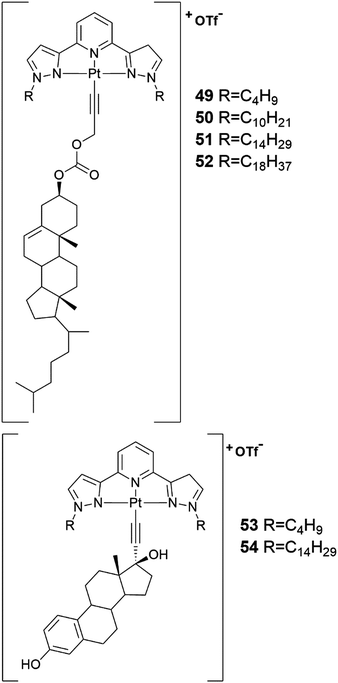 | ||
| Fig. 50 The structures of the cholesterol and estradiol derivatives containing Pt-coordinating moieties 49–54.52 | ||
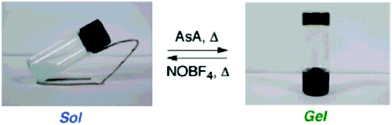
A copper complex of compound 55 (Fig. 51) formed a gel in 1-butyronitrile (1-PrCN).53 As the solution of Cu(I)·552 complex cooled and the gel formed, an unusual distinct colour change from reddish brown to greenish blue was observed (Fig. 52). The colour change occurred repeatedly, which is why the oxidation of Cu(I) to Cu(II) was excluded. Based on the UV-vis-measurements the colour change was hypothesized to originate from a distortion of the Cu(I)·552 complexes to tetrahedral coordination mode caused by gel formation. The complex was confirmed to contain two molecules of compound 55 and one Cu(I) after UV-vis-measurements and gel melting temperature tests with gels containing differing amounts of Cu-ions.
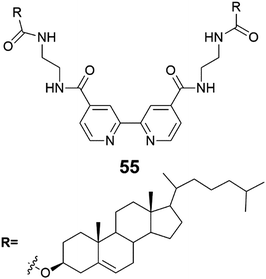 | ||
| Fig. 51 Compound 55.53 | ||
The gel of Cu(I)·552 in 1-PrCN turned into solution after adding ascorbic acid and heating following by the re-formation of a greenish blue gel during cooling.53 Addition of NOBF4 with heating resulted in a solution with a small amount of pale-blue precipitate (Fig. 53), which after adding ascorbic acid with heating formed the gel again. Based on the UV-vis-, CD-, and TEM-measurements the properties of the gel that re-formed after addition of the oxidizing agent were similar to the original gel formed by the Cu(I)·552 complex in 1-PrCN.
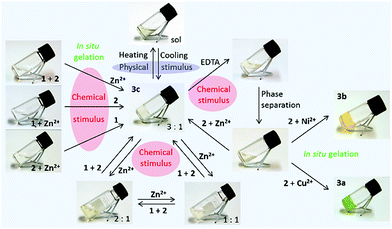
A cholesteryl amide derivative 56 was used in producing metallogels responding to multiple stimuli by subcomponent self-assembly method (Fig. 54 and 55).48 When mixing the components forming the eventual gelator in situ in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (56
1 ratio (56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ion, respectively) at room temperature a gel was formed. The mixing order was irrelevant. The metal-complexation could be seen with naked eye being colourless to green in the case of Cu(II), to yellow in the case of Ni(II), and to pale-yellow in the case of Zn(II). Gels formed also with a mixture of metal ions, provided that the overall ligand
metal ion, respectively) at room temperature a gel was formed. The mixing order was irrelevant. The metal-complexation could be seen with naked eye being colourless to green in the case of Cu(II), to yellow in the case of Ni(II), and to pale-yellow in the case of Zn(II). Gels formed also with a mixture of metal ions, provided that the overall ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ion ratio was 3
metal ion ratio was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This opens up possibilities to fine-tune the properties of the metallogel. The gels were mainly formed in alcohols, although partial gels were formed in certain other organic solvents by Ni(II)- and Zn(II)-complexes as well. The gels were transparent in 1-heptanol and 1-octanol in the case of Cu(II)- and Zn(II)-complexes, but opaque in 1-pentanol and 1-hexanol. The gels formed by the Ni(II)-complex were transparent.
1. This opens up possibilities to fine-tune the properties of the metallogel. The gels were mainly formed in alcohols, although partial gels were formed in certain other organic solvents by Ni(II)- and Zn(II)-complexes as well. The gels were transparent in 1-heptanol and 1-octanol in the case of Cu(II)- and Zn(II)-complexes, but opaque in 1-pentanol and 1-hexanol. The gels formed by the Ni(II)-complex were transparent.
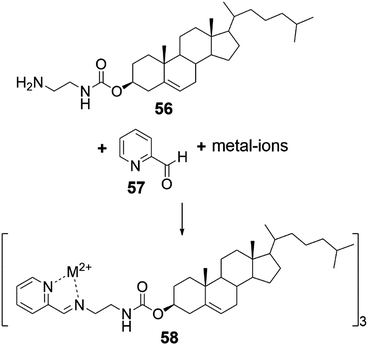 | ||
| Fig. 54 In situ preparation of the gelator 58 by subcomponent self-assembly.48 | ||
The thermal stability and strength of the gels was observed to be better in higher alcohols.48 Gels formed by Ni(II)-complex were thermally the most stable with melting temperatures of the 2% gel (w/v) in higher alcohols being >95 °C. Moreover, the Ni(II)-complex acted as a supergelator in 1-octanol, forming a gel even as low a concentration as 0.07% (w/v). The complex 58 formed thermoreversible gels in 1-octanol with each metal ion used in the study (Fig. 55, Zn(II)-complex as an example). Excess of metal-salt resulted in a collapse of the gel. Based on MS-measurements, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand
1 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal complexes did not form the gels, whereas the 3
metal complexes did not form the gels, whereas the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex acted as a gelator. After addition of a strong chelating agent (EDTA) the gel turned into a solution. After phase separation the gel could be recovered by adding metal salt, the same or different than the original one, or a mixture of metal salts providing a possibility to change the properties of the gels in a relatively simple manner when needed.
1 complex acted as a gelator. After addition of a strong chelating agent (EDTA) the gel turned into a solution. After phase separation the gel could be recovered by adding metal salt, the same or different than the original one, or a mixture of metal salts providing a possibility to change the properties of the gels in a relatively simple manner when needed.
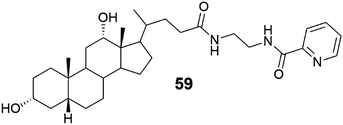 | ||
| Fig. 56 The structure of the deoxycholic acid derivative 59 capable of forming Cu(II)-metallogels.54 | ||
Deoxycholic acid derivative (Fig. 56, compound 59) was found to self-assemble into a gel only in the presence of copper salt.54 The copper salt solution was added to the aqueous solution of 59 containing methanol, acetone, or acetonitrile (30–50%). The molar ratios of 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt were 2
salt were 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. The effect of the counter ion was studied by utilizing three different salts in the gelation tests. In the case of CuCl2·2H2O only transparent blue solutions were formed. When using CuSO4·5H2O gels were formed in all ratios, predominantly during the ultrasound treatment before heating. These gels were not as stable as the gels formed with copper nitrate (Cu(NO3)2·3H2O), with which gels were formed during ultrasound treatment in ratios of 1
2, respectively. The effect of the counter ion was studied by utilizing three different salts in the gelation tests. In the case of CuCl2·2H2O only transparent blue solutions were formed. When using CuSO4·5H2O gels were formed in all ratios, predominantly during the ultrasound treatment before heating. These gels were not as stable as the gels formed with copper nitrate (Cu(NO3)2·3H2O), with which gels were formed during ultrasound treatment in ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Some of the systems studied formed a gel even the day after the heating when shaking the sample vigorously. Based on the NMR-measurements with different 59
2. Some of the systems studied formed a gel even the day after the heating when shaking the sample vigorously. Based on the NMR-measurements with different 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II) ratios, the pyridine moiety was found to be a prerequisite for the gel formation, although the gel was formed by complexes having various 59
Cu(II) ratios, the pyridine moiety was found to be a prerequisite for the gel formation, although the gel was formed by complexes having various 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II) ratios revealed by MS-measurements performed for the dissolved gel samples.
Cu(II) ratios revealed by MS-measurements performed for the dissolved gel samples.
The Cu(II)-metallogels formed by 59 collapsed as a result of adding a few drops of pyridine, triethyl amine, aquoeus ammonia, or a small amount of disodium salt of EDTA (Fig. 57).54 Redox-responsiveness of the gels was also observed (Fig. 58). Water solution of ascorbic acid afforded in reduction of the Cu(II)-ions to Cu(I)-ions indicated by the colour change of the sample. During cooling the solution turned from transparent to opaque, and after shaking the sample changed colour to green with some green precipitate. Addition of 0.5 M nitric acid with heating and ultrasound treatment re-formed the gel as a result of oxidation of the copper ions.
3 Summary and outlook
Gels are part of our everyday lives in the fields of for example cosmetics, pharmaceutics, food industry, catalysis, and coatings. Differing from the traditional polymeric gels, supramolecular gels are formed by self-assembly driven by weak interactions, such as hydrogen bonding or electrostatic, van der Waals, hydrophobic, or π–π interactions. With their reversibility and versatility supramolecular gels can be designed to respond to chemical and physical stimuli. Metal–ligand coordination may lead to metallogels possessing multi-responsive properties to wide environmental responses. Electro- or magneto-responsive metallogels have potential when designing valves, clutches, or dampers, whereas metallogels containing lanthanoids or transition metals show thermo-, mechano-, chemo-, and photo-responsiveness.55,56Particularly from the biomedical and pharmacological point of views, steroid-derived supramolecular gels with their biocompatible nature are auspicious. Steroidal metallogels may find lifesaving applications in the detection of disease markers or specific drugs. Furthermore, they may be utilized in site-specific and controlled drug delivery, cell separation, immunoassay, magnetic resonance imaging, gene delivery, minimally invasive surgery, radionuclide therapy, hyperthermia, or even artificial muscle applications.
Due to the rigid and chiral steroidal skeleton accommodating different derivatizable functional groups steroids and their derivatives have tremendous potential in forming gels with variable properties and applications. Even minuscule changes in the structure of the gelator may change not only the gelation tendencies but also the characteristics of the gels significantly. As a result, the spectrum of potential materials is extremely rich with countless potential applications waiting to be charted.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Ms Saana Rekola for updating the list of references and for Dr Hana Bunzen for the colour version of Fig. 54. University of Jyväskylä is acknowledged for financial support.References
- (a) S. B. Schryver, Proc. R. Soc. London, Ser. B, 1913, 86, 460 CrossRef; (b) S. B. Schryver, Proc. R. Soc. London, Ser. B, 1914, 87, 366 CAS; (c) S. B. Schryver, Proc. R. Soc. London, Ser. B, 1916, 89, 176 CrossRef CAS; (d) S. B. Schryver, Proc. R. Soc. London, Ser. B, 1916, 89, 361 CrossRef CAS.
- P. M. Dewick, Medicinal Natural Products: a Biosynthetic Approach, Wiley, Great-Britain, 2009, ch. 5, pp. 247–298 Search PubMed.
- V. Piironen, D. G. Lindsay, T. A. Miettinen, J. Toivo and A.-M. Lampi, J. Sci. Food Agric., 2000, 80, 939 CrossRef CAS.
- (a) J. W. Steed and J. L. Atwood, Supramol. Chem., Wiley, Great-Britain, 2009, ch. 14, pp. 888–893 Search PubMed; (b) J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC.
- (a) K. Rajangam, H. A. Behanna, M. J. Hui, X. Han, J. F. Hulvat, J. W. Lomasney and S. I. Stupp, Nano Lett., 2006, 6, 2086 CrossRef CAS PubMed; (b) V. Jayavarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611 CrossRef.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed.
- L. Mao, H. Wang, M. Tan, L. Ou, D. Kong and Z. Yang, Chem. Commun., 2012, 48, 395 RSC.
- A. Feinle, M. S. Elsaesser and N. Hüsing, Chem. Soc. Rev., 2016, 45, 3377 RSC.
- (a) K. Ghosh and D. Kar, Org. Biomol. Chem., 2012, 10, 8800 RSC; (b) K. Ghosh, D. Kar and S. Bhattacharya, Supramol. Chem., 2014, 26, 313 CrossRef CAS; (c) K. Ghosh, D. Kar, S. Panja and S. Bhattacharya, RSC Adv., 2014, 4, 3798 CAS; (d) K. Ghosh and S. Panja, RSC Adv., 2015, 5, 12094 RSC; (e) K. Ghosh, A. Panja and S. Panja, New J. Chem., 2016, 40, 3476 RSC; (f) K. Ghosh and S. Panja, ChemistrySelect, 2016, 1, 3667 CrossRef CAS; (g) Y. Wang, Z. Wang, Z. Xu, X. Yu, K. Zhao, Y. Li and X. Pang, Org. Biomol. Chem., 2016, 14, 2218 RSC; (h) K. Ghosh and S. Panja, Supramol. Chem., 2017, 29(5), 350 CrossRef CAS; (i) K. Ghosh, S. Panja and S. Bhattacharya, ChemistrySelect, 2017, 2, 959 CrossRef CAS; (j) S. Panja, S. Bhattacharya and K. Ghosh, Langmuir, 2017, 33, 8277 CrossRef CAS PubMed.
- M. O.-M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960 CrossRef CAS PubMed.
- Y. Qiao, Y. Lin, Z. Yang, H. Chen, S. Zhang, Y. Yan and J. Huang, J. Phys. Chem. B, 2010, 114, 11725 CrossRef CAS PubMed.
- J. Liu, J. Yan, X. Yuan, K. Liu, J. Peng and Y. Fang, J. Colloid Interface Sci., 2008, 318, 397 CrossRef CAS PubMed.
- (a) J. Zhang and C. Y. Su, Coord. Chem. Rev., 2010, 110, 1373 Search PubMed; (b) F. Fages, Angew. Chem., Int. Ed., 2008, 45, 1680 CrossRef PubMed; (c) T. Gorai and U. Maitra, Angew. Chem., Int. Ed., 2017, 56, 10730 CrossRef CAS PubMed.
- A. Y.-Y. Tam and V. W.-W. Yam, Chem. Soc. Rev., 2013, 42, 1540 RSC.
- For example: (a) S. Bhowmik, S. Banerjee and U. Maitra, Chem. Commun., 2010, 46, 8642 RSC; (b) S. Banerjee, R. Kandanelli, S. Bhowmik and U. Maitra, Soft Matter, 2011, 7, 8207 RSC; (c) R. Kandanelli, A. Sarkar and U. Maitra, Dalton Trans., 2013, 42, 15381 RSC; (d) R. Laishram and U. Maitra, ChemistrySelect, 2018, 3, 519 CrossRef CAS.
- For example: (a) H. Wang, S. Song, J. Hao and A. Song, Chem. – Eur. J., 2015, 21, 12194 CrossRef CAS PubMed; (b) H. Wang, W. Xu, S. Song, L. Feng, A. Song and J. Hao, J. Phys. Chem. B, 2014, 118, 4693 CrossRef CAS PubMed; (c) Y. Qiao, H. Chen, Y. Lin, Z. Yang, X. Cheng and J. Huang, J. Phys. Chem. C, 2011, 115, 7323 CrossRef CAS; (d) Y. Qiao, Y. Wang, Z. Yang, Y. Lin and J. Huang, Chem. Mater., 2011, 23, 1182 CrossRef CAS.
- A. Chakrabarty, U. Maitra and A. D. Das, J. Mater. Chem., 2012, 22, 18268 RSC.
- M. Maity and U. Maitra, J. Mater. Chem. A, 2014, 2, 18952 RSC.
- S. Bhowmik, T. Gorai and U. Maitra, J. Mater. Chem. C, 2014, 2, 1597 RSC.
- (a) R. Laishram and U. Maitra, Asian J. Org. Chem., 2017, 6, 1235 CrossRef CAS; (b) T. Gorai and U. Maitra, J. Mater. Chem. B, 2018, 6, 2143 RSC; (c) S. Bhowmik and U. Maitra, Chem. Commun., 2012, 48, 4624 RSC.
- H. Wang, W. Xu, S. Song, L. Feng, A. Song and J. Hao, J. Phys. Chem. B, 2015, 118, 4639 Search PubMed.
- R. Laishram, S. Bhowmik and U. Maitra, J. Mater. Chem. C, 2015, 3, 5885 RSC.
- (a) J. C. Medina, I. Gay, Z. Chen, L. Echegoyen and G. W. Gokel, J. Am. Chem. Soc., 1991, 113, 365 CrossRef CAS; (b) K. Wang, S. Muños, L. Zhang, R. Castro, A. E. Kaifer and G. W. Gokel, J. Am. Chem. Soc., 1996, 118, 6707 CrossRef CAS.
- T. Klawonn, A. Gansäuer, I. Winkler, T. Lauterbach, D. Franke, R. J. M. Nolte, M. C. Feiters, H. Börner, J. Hentschel and K. H. Dötz, Chem. Commun., 2007, 1894 RSC.
- (a) J. Liu, P. He, J. Yan, X. Fang, J. Peng, K. Liu and Y. Fang, Adv. Mater., 2008, 20, 2508 CrossRef CAS; (b) P. He, J. Liu, K. Liu, L. Ding, J. Yan, D. Gao and Y. Fang, Colloids Surf., A, 2010, 362, 127 CrossRef CAS; (c) J. Yan, J. Liu, Y. Sun, P. Jing, P. He, D. Gao and Y. Fang, J. Phys. Chem. B, 2010, 114, 13116 CrossRef CAS PubMed.
- C. E. Housecroft and A. G. Sharpe, Inorganic Chemistry, Pearson Education Limited, England, 2008, ch. 24, pp. 841–845 Search PubMed.
- A. Gansäuer, I. Winkler., T. Klawonn, R. J. M. Nolte, M. C. Feiters, H. G. Börner, J. Hentschel and K. H. Dötz, Organometallics, 2009, 28, 1377 CrossRef.
- (a) V. S. Sajisha and U. Maitra, Chimia, 2013, 67, 44 CrossRef CAS PubMed; (b) Y. Okamoto and J. Kido, Chem. Rev., 2002, 102, 2357 CrossRef PubMed.
- (a) M. Maity and U. Maitra, Dalton Trans., 2017, 46, 9266 RSC; (b) Y. Qiao, Y. Lin, Y. Wang, Z. Yang, J. Liu, J. Zhou, Y. Yan and J. Huang, Nano Lett., 2009, 9(12), 4500 CrossRef CAS PubMed.
- (a) S. Chatterjee and U. Maitra, Nanoscale, 2016, 8, 14979 RSC; (b) S. Chatterjee and U. Maitra, Nanoscale, 2017, 9, 13820 RSC.
- T. Gorai and U. Maitra, ACS Sens., 2016, 1, 934 CrossRef CAS.
- Y. Qiao, Y. Lin, S. Zhang and J. Huang, Chem. – Eur. J., 2011, 17, 5180 CrossRef CAS PubMed.
- Y. Wang, X. Xin, W. Li, C. Jia, L. Wang, J. Shen and G. Xu, J. Colloid Interface Sci., 2014, 431, 82 CrossRef CAS PubMed.
- R. Laishram and U. Maitra, Chem. – Asian J., 2017, 12, 1267 CrossRef CAS PubMed.
- (a) N. M. Sangeetha, R. Balasubramanian, U. Maitra, S. Ghosh and A. R. Raju, Langmuir, 2002, 18, 7154 CrossRef CAS; (b) N. M. Sangeetha, S. Bhat, A. Choudhury, U. Maitra and P. Terech, J. Phys. Chem. B, 2004, 108, 16056 CrossRef CAS; (c) S. Bhat and U. Maitra, Tetrahedron, 2007, 63, 7309 CrossRef CAS.
- For example: (a) V. Noponen, Nonappa, M. Lahtinen, A. Valkonen, H. Salo, E. Kolehmainen and E. Sievänen, Soft Matter, 2010, 6, 3789 RSC; (b) M. Löfman, J. Koivukorpi, V. Noponen, H. Salo and E. Sievänen, J. Colloid Interface Sci., 2011, 360, 633 CrossRef PubMed; (c) V. Noponen, H. Belt, M. Lahtinen, A. Valkonen, H. Salo, J. Ulrichová, A. Galandáková and E. Sievänen, Steroids, 2012, 77, 193 CrossRef CAS PubMed; (d) V. Noponen, A. Valkonen, M. Lahtinen, H. Salo and E. Sievänen, Supramol. Chem., 2013, 25, 133 CrossRef CAS; (e) R. Kuosmanen, R. Puttreddy, R.-M. Willman, I. Äijäläinen, A. Galandáková, J. Ulrichová, H. Salo, K. Rissanen and E. Sievänen, Steroids, 2016, 108, 7 CrossRef CAS PubMed.
- J.-S. Shen, Y.-L. Chen, J.-L. Huang, J.-D. Chen, C. Zhao, Y.-Q. Zheng, T. Yu, Y. Yang and H.-W. Zhang, Soft Matter, 2013, 9, 2017 RSC.
- B. Kuppan and U. Maitra, ChemNanoMat, 2018, 4, 846 CrossRef CAS.
- T. Tu, W. Fang, X. Bao, X. Li and K. H. Dötz, Angew. Chem., Int. Ed., 2011, 50, 6601 CrossRef CAS PubMed.
- Y. Li, A. Y.-Y. Tam, K. M.-C. Wong, W. Li, L. Wu and V. W.-W. Yam, Chem. – Eur. J., 2011, 17, 8048 CrossRef CAS.
- K. Liu, L. Meng, S. Mo, M. Zhang, Y. Mao, X. Cao, C. Huang and T. Yi, J. Mater. Chem. C, 2013, 1, 1753 RSC.
- M. Dukh, D. Šaman, J. Kroulik, I. Černý, V. Pouzar, V. Král and P. Drašar, Tetrahedron, 2003, 59, 4069 CrossRef CAS.
- S. Malik, S.-I. Kawano, N. Fujita and S. Shinkai, Tetrahedron, 2007, 63, 7326 CrossRef CAS.
- A. Panja and K. Ghosh, Mater. Chem. Front., 2018, 2, 2286 RSC.
- G. Liu, J. Sheng, W. L. Teo, G. Yang, H. Wu, Y. Li and Y. Zhao, J. Am. Chem. Soc., 2018, 140, 16275 CrossRef CAS PubMed.
- S. Debnath, J.-F. Bergamini, F. Artzner, C. Mériadec, F. Camerel and M. Fourmigué, Chem. Commun., 2012, 48, 2283 RSC.
- K. Murata, M. Aoki, T. Nishi, A. Ikeda and S. Shinkai, J. Chem. Soc., Chem. Commun., 1991, 0, 1715 RSC.
- H. Bunzen, Nonappa, E. Kalenius, S. Hietala and E. Kolehmainen, Chem. – Eur. J., 2013, 19, 12978 CrossRef CAS PubMed.
- T. Ishi-i, R. Iguchi, E. Snip, M. Ikeda and S. Shinkai, Langmuir, 2001, 17, 5825 CrossRef CAS.
- D. D. Díaz, J. J. Cid, P. Vázquez and T. Torres, Chem. – Eur. J., 2008, 14, 9261 CrossRef PubMed.
- P. Terech and R. G. Weiss, Molecular Gels – Materials with Self-Assembled Fibrillar Networks, Springer, Netherlands, 2006, ch. 27, pp. 895–927 Search PubMed.
- Y. Li, E. S.-H. Lam, A. Y.-Y. Tam, K. M. C. Wong, W. H. Lam, L. Wu and V. W.-W. Yam, Chem. – Eur. J., 2013, 19, 9987 CrossRef CAS PubMed.
- S.-i. Kawano, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2004, 126, 8592 CrossRef CAS PubMed.
- V. Noponen, K. Toikkanen, E. Kalenius, R. Kuosmanen, H. Salo and E. Sievänen, Steroids, 2015, 97, 54 CrossRef CAS PubMed.
- B. Xing, M. Choi and B. Xu, Chem. – Eur. J., 2002, 8, 5028 CrossRef CAS.
- W. Weng, J. Beck, A. Jamieson and S. Rowan, J. Am. Chem. Soc., 2006, 128, 11663 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |