On-chip electrocatalytic microdevice: an emerging platform for expanding the insight into electrochemical processes
Abstract
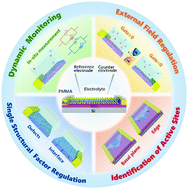
Electrochemical conversion is an important process in renewable energy conversion, and electrocatalysts play a vital role since they can improve the rate and efficiency of chemical transformations. Thus, the continuing interest in electrocatalysis is fueled both in terms of mechanism exploration and performance optimization, and this field is continuously being updated. However, conventional electrochemical methods still have room to be explored, such as in situ dynamic monitoring, external field regulation, and single-entity electrocatalytic detection. Noteworthily, inspired by the recent success in nanoelectronic semiconductor devices, the emerging field of on-chip electrocatalytic microdevices, focusing on the electrochemical behaviors at individual nanowire/nanosheet as the working electrode, has emerged as a powerful alternative platform to the traditional techniques. This unique device configuration enables several advantages, such as in situ electronic/electrochemical measurements and adjustable microstructure of individual catalysts, which is constantly expanded to directly probe electrochemical processes to obtain previously inaccessible information. Hence, herein, we first introduce the device configuration and its advantages as an emerging platform. Subsequently, the attempts to expand the insight into electrochemical processes through this type of microdevice are explicitly analyzed and summarized including dynamic monitoring, external field regulation, identification of active sites, and single structural factor regulation. Finally, some personal perspectives on the challenges and future research directions in this promising area are also presented. We believe that this review will provide new insight into electrochemical processes, ranging from dynamic exploration to performance optimization.


 Please wait while we load your content...
Please wait while we load your content...