The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer
Pui-Chi
Lo
 a,
M. Salomé
Rodríguez-Morgade
a,
M. Salomé
Rodríguez-Morgade
 bc,
Ravindra K.
Pandey
bc,
Ravindra K.
Pandey
 *d,
Dennis K. P.
Ng
*d,
Dennis K. P.
Ng
 *e,
Tomás
Torres
*e,
Tomás
Torres
 *bcf and
Fabienne
Dumoulin
*bcf and
Fabienne
Dumoulin
 *g
*g
aDepartment of Biomedical Sciences, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, China
bDepartamento de Quimica Orgánica, Universidad Autonoma de Madrid (UAM), Cantoblanco, 28049 Madrid, Spain. E-mail: tomas.torres@uam.es
cInstitute for Advanced Research in Chemical Sciences (IAdChem), UAM, 28049 Madrid, Spain
dPhotodynamic Therapy Center, Cell Stress Biology, Roswell Park Cancer Institute, Buffalo, NY, USA. E-mail: ravindra.pandey@roswellpark.org
eDepartment of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China. E-mail: dkpn@cuhk.edu.hk
fIMDEA Nanociencia, Cantoblanco 28049, Madrid, Spain
gGebze Technical University, Chemistry Department, Gebze, 41400 Kocaeli, Turkey. E-mail: fdumoulin@gtu.edu.tr; dumoulin@gmail.com
First published on 17th December 2019
Abstract
Phthalocyanines exhibit superior photoproperties that make them a surely attractive class of photosensitisers for photodynamic therapy of cancer. Several derivatives are at various phases of clinical trials, and efforts have been put continuously to improve their photodynamic efficacy. To this end, various strategies have been applied to develop advanced phthalocyanines with optimised photoproperties, dual therapeutic actions, tumour-targeting properties and/or specific activation at tumour sites. The advantageous properties and potential of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer are highlighted in this tutorial review.
Key learning points(1) The advantages of phthalocyanines over other classes of photosensitisers(2) Phthalocyanines engaged in clinical trials (3) Strategies to improve the photodynamic efficacy of phthalocyanines, including their intriguing photoproperties, tumour-targeting and site-specific activation, dual therapeutic actions and theranostics (4) Molecular and nano-engineering of phthalocyanine-based photosensitisers for photodynamic therapy |
1. Introduction
Most of the porphyrin-based compounds including those currently approved for the treatment of a variety of cancers (e.g., Photofrin and Foscan) are associated with skin phototoxicity, which led to exploring the utility of phthalocyanine (Pc) and related naphthalocyanine systems. These compounds exhibit long wavelength absorptions and fluorescence in the range of 650 to 800 nm with a high singlet oxygen producing ability for the destruction of superficial and deep-seated tumours. Unlike porphyrins, these structures exhibit weak absorption in the range of 400–600 nm, which minimises skin phototoxicity. Similar to porphyrins, Pc systems are also capable of forming metal complexes, which further increase their singlet oxygen producing ability, one of the key requirements of the photodynamic therapy (PDT) process.A list of phthalocyanine-based photosensitisers (PSs) have been in clinical trials. For example, CGP55847, a liposomal formulation of unsubstituted ZnPc developed by Ciba-Geigy,1 underwent Phase I/II clinical trials against squamous cell carcinomas of the upper aerodigestive tract, but no human clinical trial data have been reported yet. Photosens,2 a Pc derivative containing aluminium as the central metal, was developed in Russia and evaluated for the treatment of skin, breast, gastro-intestinal and lung malignancies, as well as neovascularization of the eye. The water solubility and bioavailability of the molecule were increased by introducing sulfonic acid groups in the benzene moiety present at the periphery of the molecule. Among the Pc-based compounds, aluminium tetra-sulfonated Pc was investigated in trials due to its desired photophysical properties and water solubility. Unfortunately, in pre-clinical studies, this compound showed neurotoxicity in rabbits, which could be limited by reducing the number of sulfonic groups from four to two. Interestingly, in preclinical studies, the sulfonic groups present in the adjacent isoindole subunits of the Pc (AlPcS2adj derivative) were more effective than those introduced at opposite positions, and proved to be a powerful photochemical internalisation (PCI) agent.3Photocyanine, an isomeric mixture of di-(potassium sulfonate)-di-phthalimidomethyl ZnPc (Fig. 1), has also reached Phase II clinical trials in China, and proved to be effective against human hepatocellular carcinoma HepG2.4
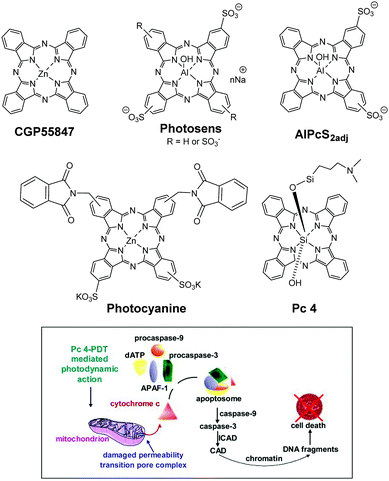 | ||
| Fig. 1 Top: Structures of Pcs approved or having been or being enrolled in human clinical trials of cancer by PDT. Bottom: Main mechanism of the induction of apoptosis by Pc 4-PDT in vitro. Reproduced from ref. 5 with permission from Elsevier. | ||
Among the Pcs evaluated so far in the clinic, Pc 4, a silicon-based metallophthalocyanine5 (Fig. 1) developed by Vitex, USA, has shown the greatest potential in breast, human colon and ovarian cancers. Oleinick and coworkers also evaluated the utility of Pc 4 for the treatment of cutaneous T-cell lymphoma (CTCL). The inventors developed an immunohistochemical assay for caspase-3 activation, using biopsies from patients treated with topical Pc 4 in Phase I trials. Results suggest that this assay may be used as an early stage biomarker of clinical response. The objectives of this clinical study were to evaluate the mechanism of cell killing by Pc-PDT. The preliminary work suggests (a) cardiolipin and the anti-apoptotic proteins Bcl-2 and Bcl-xl as the targets of therapy and (b) the apoptosis with the participation of caspase-3 in the central response to Pc-PDT. This is an ongoing trial, and thirty patients have been treated so far. No local or systemic toxicities have been reported. The responders and non-responders tolerated the light treatment well, with only minor tingling sensation during laser irradiation.
For accessible lesions, topical application is a convenient and useful approach for directing PSs, avoiding unwanted distribution of most of the PSs after intravenous injection, and has been well studied with protoporphyrin IX prodrug ALA and its esters. So far, most of the tumour-avid porphyrin-based PSs showed limited benefit in their topical application. However, the clinical results discussed by Oleinick et al. indicate that topically applied Pc-4 can be effective for CTCL, and possibly for other topical applications of a variety of cancer types.
Problems and future directions
Some of the Pcs, when exposed to light, produce photodegradation products (metabolites), which may produce organ toxicity. However, attempts are underway to overcome these problems by incorporating them in suitable biodegradable/biocompatible nanoparticles. This approach has been extremely promising in developing multifunctional nanoplatforms for tumour targeting agents with enhanced tumour-specificity. These nanoconstructs have also shown great promise for both cancer-imaging and therapy.Many efforts have been devoted to improving and optimising the photodynamic efficiency of Pcs used in PDT of cancer. The delay in clinical trials of various promising PDT agents, including Pcs, has been mainly due to limited financial support. After the commercialization of Photofrin, a number of superior agents (including Pcs) have shown promising potential. Unfortunately, due to the limited financial support from big pharmaceutical companies, it has been difficult to complete the Phase II/III clinical trials of these agents, which are extremely expensive.
This review summarises the most recent developments in this field, including studies with at least in vitro data. Emphasis will also be put on the various strategies to enhance the functions of Pcs, showing the great promise of this class of functional dyes as advanced PSs for PDT of cancer.
2. Optimisation of photophysical properties and biocompatibility
Pcs can coordinate more than 70 elements of the periodic table. However, not all Pc complexes are suitable to generate reactive oxygen species, necessary for PDT. The optimisation of their photophysical properties is crucial to the success of the treatment. For instance, free-base Pcs generally display lower singlet oxygen quantum yields when compared with the corresponding ZnPcs.6 Pcs complexing ions functionalisable at their axial positions such as Si4+, Al3+ and Ga3+ are also often preferred, since closed-shell diamagnetic ions provide the resulting MPcs with high-yield and long-lived triplet states, while axial functionalisation reduces aggregation. The heavy atom effect (heavy metals or halogens) resulting from the enhanced spin–orbit coupling between the metal ion d-orbitals and the Pc π-system can enhance the SOG. Therefore, metal complexes exhibit a greater tendency to undergo intersystem crossing (ISC) from the singlet excited state S1 to the triplet excited state T1, which is reflected by high triplet state quantum yields. In this respect, Ru2+ produces complexes with high triplet state quantum yields and provides two axial coordination sites that allow preparing robust complexes. In contrast, metals with a paramagnetic nature like Fe, Cu and Gd are not suitable for application as PSs.The usually poor solubility of Pcs in biological aqueous media prevents their biological application, or at least induces detrimental aggregation, which inhibits ROS generation. The planar, aromatic structure of Pcs, responsible for their photophysical properties, causes an important disadvantage in biological media, since it gives rise to their low solubility in water and to their strong tendency to form aggregates. The aggregation states, which result from π-stacking of macrocycles, are enhanced in aqueous media, owing to the hydrophobic effect. On one hand, the administration of drugs with poor solubility in water results in low bioavailability, aggregation upon intravenous administration, or toxic effects derived from their local accumulation. Therefore, several strategies are reported to enhance Pcs’ water-solubility.7 On the other hand, too hydrophilic drugs are unable to cross the amphiphilic cellular membrane, resulting in poor cellular uptake and reduced stability against proteolytic and hydrolytic degradation. Functionalisation of Pcs, either at their peripheral or axial positions, serves to provide solubility, modulate photophysical parameters, or introduce functionalized or targeting moieties. The biocompatibility of Pcs can be enhanced through their functionalisation with ionic functions, or with hydrophilic groups, such as peptides, polyethers (PEG) and carbohydrates. In this respect, the design of amphiphilic photosensitisers with good hydrophilic–hydrophobic balance should lead to good localization at the interfaces of the cellular membranes and at the surfaces of proteins.
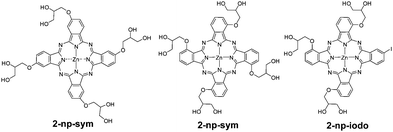
The substitution with PEG chains is a common strategy to increase the hydrophilicity of Pcs. PEG comprises a chemically inert structure with the general formula XO–(CH2CH2O)n–CH2CH2OX. A recent example of PEGylation of RuPcs at their axial positions has been reported (Fig. 2).8,9 The functionalisation at the axial positions plays the dual role of introducing a physical property, such as solubility in this case, as well as preventing aggregation. Here, the number and length of PEG chains per macrocycle is crucial in order to modulate the solubility properties. However, higher hydrophilicity does not necessarily lead to higher uptake, and higher uptake does not automatically mean higher photodynamic efficiency. In fact, in this study, the most hydrophilic compound Ru(L3)2Pc (Fig. 2) shows the lowest cellular uptake, although the highest phototoxicity, pointing to a more favourable intracellular localization of this Pc. Polyether chains have also been introduced at the axial positions of SiPcs, affording compounds with enhanced hydrophilicity, reduced aggregation and high in vitro phototoxic effects against human bladder HT-1376 cancer cells. Usually, functionalisation at the phthalocyanine periphery produces stronger alteration of the photophysical parameters, but, compared to axial substitution, is less efficient to reduce aggregation in water. An example is constituted by water soluble, glycerol-substituted ZnPcs of type 2 (Fig. 3).10 The attachment of these electron-donating alkoxy substituents red shifts the phthalocyanine Q-bands to 680–704 nm, but does not prevent aggregation. Comparing peripheral- and non-peripheral-substitution, the latter (like in 2-np-sym and 2-np-iodo) provides larger red shifts, and slightly less aggregated species. The more hydrophilic tetra-glycerol substituted Pcs show lower cellular uptake in MCF-7, HCT-116 and HSC-2 cancer cells, compared to tri-glycerol-substituted derivative 2-np-iodo. Compared to the tetrasulfonated ZnPc used as the reference, 2-np-sym and 2-np-iodo proved to induce efficient photodynamic damage to chick embryo chorioallantoic membrane blood vessels, reflecting strong potential for antivascular PDT.
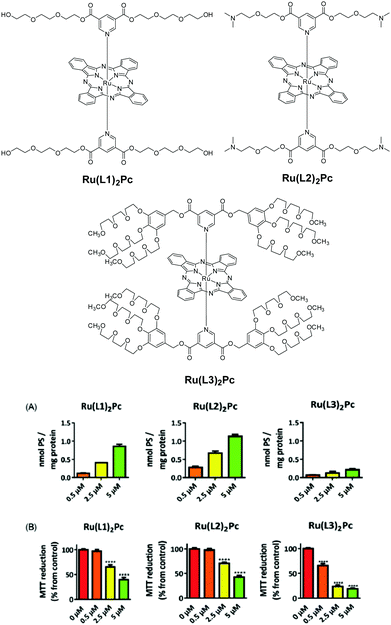 | ||
| Fig. 2 Top: RuPcs with various axial PEG substitutions. Bottom: Cellular uptake by HT-1376 cells after 2 h incubation (A), and phototoxicity evaluated 24 h after incubation with RuL2Pcs for 2 h and irradiation for 40 min at a fluence rate of 20 mW cm−2 (B). Reproduced from ref. 8 with the permission of The Royal Society of Chemistry. | ||
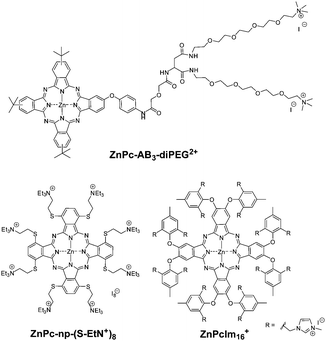
Another example of peripheral PEGylation of Pcs uses these functions to connect an ammonium-based, dicationic entity to the B unit of amphiphilic ZnPcs of A3B type (ZnPc-AB3-diPEG2+, Fig. 4).11 Charged substituents are expected to induce disaggregation by electrostatic repulsion forces. ZnPc-AB3-diPEG2+ was substituted with a peripheral aryloxy moiety containing PEG chains that are not directly attached to the Pc core. The combination of two polyether and two ionic functions does not provide solubility in neat water, but only in solutions containing 1% of DMF or DMSO. ZnPc-AB3-diPEG2+ show good phototoxicity against human carcinoma HEp2 cells, presumably due to their intracellular localization in the endoplasmic reticulum, rather than the extent of their cellular uptake, which is also low in this case. On the other hand, suppression of the aggregation in aqueous media is not complete in this case, since the charged substituents are freely pending, and π–π stacking of macrocycles is still possible.11
Similar studies performed on ammonium-based, octa-cationic ZnPc-np-(S-EtN+)8, connected to macrocycles through flexible alkylsulfanyl functions, illustrate once more the inability of the ionic functions to completely prevent by themselves macrocycle aggregation in water.6 The octa-cationic character of the macrocycles is enough to provide high solubility in water. Again, non-peripheral substitution produces larger red shifts (ca. 80 nm more) of the Q-band compared to peripheral substitution and better reduction of aggregation. Compared to the Mg analogue, the Zn derivative has a much better SOG thanks to the heavy atom effect of the Zn. Compared to the peripherally substituted analogue, the SOG quantum yield of ZnPc-np-(S-EtN+)8 was much lower (0.20 vs. 0.91 in DMF). Tested against 3T3, HeLa, SK-MEL-28, and HCT 116 cancer cells, ZnPc-np-(S-EtN+)8 was slightly more toxic in the dark than its peripheral counterpart and less efficient from a photodynamic point of view. However, the IC50 values remained low and ZnPc-np-(S-EtN+)8 has the indisputable advantage of allowing excitation at longer wavelengths.
Aggregation in water can be suppressed smoothly by combination of ionic functions in bulky and rigid peripheral substituents. In this respect, pyridinium, imidazolium, quinolinium or morpholinium salts provide cationic and rigid systems. The hexadeca-cationic ZnPc-Im16+ (Fig. 4), containing imidazolium rings, represents an example of an excellent water-soluble, non-aggregated photosensitiser.12 Based on the crystal structure of its corresponding phthalonitrile precursor, ZnPcIm16+ is very likely to have the eight aryloxy groups arranged in an orthogonal disposition with respect to the ZnPc plane, with cationic imidazolium rings situated over and below the macrocycle, in a rigid configuration that inhibits π–π stacking. No dark toxicity was detected on 3T3 cells (non-malignant mouse fibroblast cell line, TC50 395 μM) and HeLa cells (human cervical carcinoma cell line, TC50 628 μM), while photodynamic EC50 was as low as 36.7 nM, evidencing the strong potential of this compound, which was distributed mainly in lysosomes.
Other ways to improve the biocompatibility of Pcs use covalent and amphiphilic combinations of α, β, or γ-cyclodextrins (CDs) with lipophilic phthalocyanines (ZnPc-α-CD, Fig. 5).13 The resulting conjugates are of AABB type, suitable for good cell penetration. These compounds have enhanced solubility in water, and exhibit different aggregation behaviours in PBS, depending on the type of CD. In particular, the higher tendency of β-CD to self-aggregate leads to lower solubility, singlet oxygen generation, cellular uptake and phototoxic activity. In contrast, α- and γ-CD conjugates exhibit powerful phototoxicity against UM-UC-3 human bladder cancer cells, with submicromolar IC50 values upon red light irradiation.
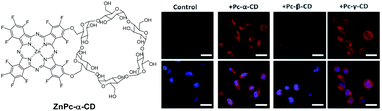 | ||
| Fig. 5 Left: Structure of the ZnPc-α-CD conjugate. Right: Fluorescence after incubation of ZnPc–CDs in UM-UC-3 bladder cancer cells in the dark (red, top) and after DAPI staining of the cell nuclei (blue, bottom). Scale bars 20 mm. Adapted from ref. 13 by permission of The Royal Society of Chemistry. | ||
The bioavailability of PSs can also be achieved through their formulation/encapsulation in Cremophor, liposomes,14 micelles,15 nanoparticles, bovine serum albumin (BSA), low-density lipoprotein (LDL), peptides, or inclusion in supramolecular assemblies. This allows using some lipophilic Pcs that might show superior photophysical parameters and/or enhanced tumour-to-normal tissue ratios, but that cannot be delivered otherwise. For example, unsubstituted ZnPc in liposomal formulation (CGP55847) has been considered a promising candidate for clinical PDT. The incorporation of PEG moieties in nanoparticles for the delivery of hydrophobic drugs, as well as their use as blocks for the formation of carriers based on polymeric micelles, has also been described.
However, monofunctionalised A3B ZnPc has been conjugated to biodegradable, polymeric poly-L-glutamic acid (PGA), to afford 1-PG in good yields (Fig. 6). Its cellular uptake and phototoxicity have been compared with the corresponding free, non-conjugated ZnPc. The conjugation with PGA not only increases the cellular uptake 15-fold (via clathrin-mediated endocytosis), but also enhances singlet oxygen generation in DMSO.16 The conjugate remains mainly in the cytoplasm (Fig. 5). Further in vivo experiments17 on Wistar-Kyoto rats showed quasi-total (91%) tumour volume reduction and tumour accumulation, and an excellent cardiovascular safety profile without metastatic propagation.
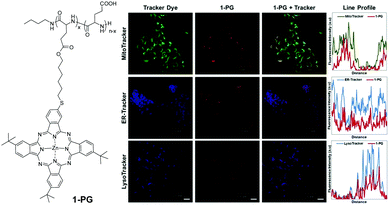 | ||
| Fig. 6 Left: Structure of ZnPc–polymeric poly-L-glutamic acid conjugate 1-PG. Right: Co-stained experiments with LysoTracker, ER-Tracker, and MitoTracker in 4T1 cells. Scale bars: 20 μm. Adapted from ref. 16 with the permission from Elsevier. | ||
Triblock (PEO–PPO–PEO) copolymers registered under the trademark of Pluronics® are able to self-assemble in water into micelles, allowing the delivery of poorly soluble compounds. These vehicles, composed of two hydrophilic polyethylene oxide (PEO) blocks and one lipophilic polypropylene oxide (PPO) fragment, can be used to administer both lipophilic and hydrophilic drugs, by increasing the solubility in water of the former, or decreasing the high solubility of the latter, as it would be responsible for their rapid excretion. Pluronic formulations of two analogous ZnPcs, one octasubstituted with monomethylether diethyleneglycol chains and the other with the corresponding fluorinated chains, have been prepared.18 The presence of the fluorinated chains had the double advantage of enhancing SOG thanks to the heavy atom effect and the affinity of fluorine atoms for oxygen. Upon addition of the Pluronic formulations, the macrocycles were solubilized in a non-aggregated form, showing a stronger photodynamic effect than the non-formulated Pc, and reaching 90% mortality, in contrast to free Pc (50% mortality). The formulations enhanced also their cellular uptake in A549, CT26 and 2H11 cells. Improved biodistribution and photodynamic efficacy were demonstrated in vivo in CT26 bearing BALB/c mice. The fluorinated Pc induced complete tumour regression in 20% of the treated mice without relapse for over one year after treatment.
Incorporation into supramolecular self-assemblies is another way to improve the biocompatibility and photodynamic efficiency of Pcs. The aggregation of AB3 type ZnPc(COOH)12-diAd, which is dodecacarboxylated and also bears two adamantane units19 (Fig. 7), is inhibited by host–guest supramolecular interactions with β-CD. The latter, which constitutes a biochemical carrier bearing alkyl chains on its secondary face and oligoethylene glycol units on its primary face, formed unilamellar CD vesicles (CDVs) in aqueous media (Fig. 7). This allows immobilization of the Pc on the surfaces of the vesicles. The adamantane–CD host–guest interactions compensate for the free energy of the hydrophobic effect, thus preventing macrocycle π–π stacking.
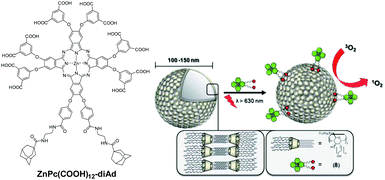 | ||
| Fig. 7 Structure of hexadeca-ionic di-adamantane ZnPc 6 and its immobilization at the surfaces of CDVs. Reproduced from ref. 19 by permission of The Royal Society of Chemistry. | ||
A recent example reports on the formation of SCMP(ZnPc)-HCCP nanoparticles as near-infrared PSs. These nanoparticles consist of a soluble conjugated microporous polymer, made of a cyclomatrix polyphosphazene porous network, which is covalently multifunctionalized with ZnPcs (Fig. 8).20 The nanoparticles are formed in water in the presence of polyvinylpyrrolidone (PVP), so that the hydrophobic Pc-containing polyphosphazene is placed in the core, with the PVP situated outside, forming a corona. The Pcs are J-aggregated, and this assembly produces a broadened and impressive bathochromic NIR shift of the Pc absorption (Fig. 8). When excited at 785 nm with a NIR laser, dual photothermal ablation and photodynamic effects (PTT-PDT) are produced, with remarkable killing of HepG2 cells.
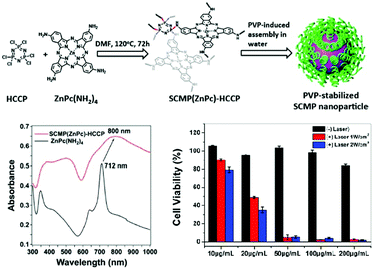 | ||
| Fig. 8 Top: Preparation of PVP-stabilized SCMP(ZnPc)-HCCP nanoparticles. Bottom left: UV-vis-NIR spectra of ZnPc(NH2)4 and SCMP(ZnPc)-HCCP in THF. Bottom right: Cell viabilities of HepG2 cells at varying concentrations of PVP-stabilized SCMP(ZnPc)-HCCP nanoparticles, without (black) or with 785 nm irradiation (red: 1 W cm−2, blue: 2 W cm−2). Adapted with permission from ref. 20. Copyright 2018 American Society of Chemistry. | ||
3. Non-conventional excitation modes
PDT involves administration of a PS followed by irradiation at an appropriate wavelength, preferentially at its maximum absorption. Most of the PSs commonly used for this purpose need continuous activation by visible light, and the limited penetration depth of the light in the tissues could represent a problem. Several non-conventional excitation modes have been developed for overcoming this limitation. Excitation at NIR (near infrared) wavelengths is preferred for two reasons: (a) the longer the wavelength, the deeper the light penetration into tissues, and (b) NIR wavelengths do not excite endogenous chromophores such as haemoglobin. Optimal excitation is hence done in the so-called phototherapeutic NIR window, located between 650 and 1000 nm. Other phototherapeutic windows with longer wavelengths are reported.The combination of upconversion NPs (UCNPs) and PSs is one of the options to excite with longer wavelengths. These nanoparticles, when irradiated with NIR light at 980 nm, emit visible luminescence at a shorter wavelength that can activate the Pc. NaYF4:Yb3+/Er3+@SiO2 nanospheres (NaYF4:Yb3+/Er3+ nanospheres coated with a thin shell of homogenous silica) were further functionalized with branched polyethyleneimine (PEI), allowing covalent grafting of an octacarboxylated AlPc (AlPc(COOH)8, Fig. 9) onto their surfaces, then a final coating with a succinimidyl ester of poly-PEG is added to improve their biocompatibility.21 The excitation at 980 nm of these NaYF4:Yb3+/Er3+@SiO2-PEI-Pc-PEG NPs was fully transferred to the AlPc, which generated 1O2. Administration to mammary carcinoma MDA-MB-231 cells growing subcutaneously in athymic nude mice resulted in a partial-to-complete eradication of the tumour.
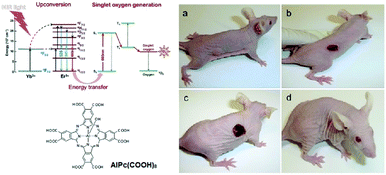 | ||
| Fig. 9 Left top: Schematic illustration of NIR-triggered photodynamic therapy using NaYF4:Yb3+/Er3+@SiO2-PEI-Pc-PEG nanoparticles. Left bottom: Structure of the octacarboxy AlPc. Right: Nu/nu mouse with growing human mammary carcinoma MDAMB-231 before therapy (a). Development of necrosis post-PDT with NaYF4:Yb3+/Er3+@SiO2-PEI-Pc-PEG: 2 (b) and 5 (c) days after PDT. The completely healed mouse 20 days after PDT (d). Adapted from ref. 21 with the permission of Wiley. | ||
However, an important drawback of excitation at 980 nm lies in the high absorption of the laser light by water in the tissues and the possible overheating in case of long-term continuous irradiation, plus the absorption by water which decreases the excitation efficiency. The use of lower wavelength NIR light can improve the performance of these systems, affording a better penetrability. Excitation at 808 nm appears in this respect to be more suitable. Recently, renewable NIR persistent luminescence sensitised PDT has been reported as a non-conventional excitation mode that provides an internal light source able to generate 1O2. Some types of nanoparticles can store excitation energy and then slowly emit it as persistent luminescence for a long time, even hours after the external excitation source ceases. Interestingly, these systems work like an optical battery and the storage of energy can be achieved, e.g., by means of UV-vis light or X-rays. Yan and co-workers recently explored Cr3+ doped zinc gallogermanate nanoparticles (ZGGO:Cr3+) as persistent luminescent nanoparticles (PLNPs), taking advantage of their 808 nm light renewable NIR persistent luminescence. In this particular case, the PDT nanoplatform was constructed by using a SiPc as the PS, which was covalently conjugated onto the surfaces of the amino-functionalized nanoparticles, giving Si-Pc–PLNPs (Fig. 10).22 MTT assays on 293T cells (a human embryonic kidney cell line) and HepG2 cells (a hepatocellular carcinoma cell line) revealed a very low dark toxicity of these constructs, and confirmed that the phototoxicity was induced by the persistent luminescence sensitised PDT effect. In vivo PDT experiments conducted on subcutaneous HepG2 tumours grown in Balb/c mouse models showed that the tumour volumes of the mice treated with the pre-excited Si-Pc–PLNPs and irradiated with 808 nm significantly decreased over time, whereas those of control mice continued to grow. The authors stated that this work provides a new way to design new nanoplatforms for highly efficient PDT, without any need for continuous external light irradiation.
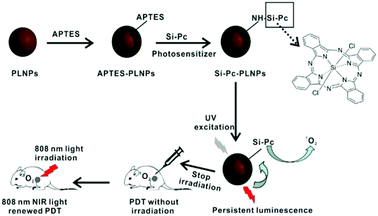 | ||
| Fig. 10 Preparation of an 808 nm NIR light renewable persistent luminescence sensitised PDT platform and administration to mice. Reproduced from ref. 22 by permission of The Royal Society of Chemistry. | ||
X-ray induced PDT, or X-PDT, modes have also been reported.23,24 The base of this technology comprised an integrated nanosystem, consisting of a nanoparticle scintillator core, which is loaded with a PS whose excitation matches the emission of the scintillator. It transforms X-ray photons into visible ones, a phenomenon known as X-ray excited optical luminescence (XEOL). In this way, activation of the PS takes place, giving rise to 1O2 generation. This new modality of therapy holds great potential to treat deep-seated tumours in internal organs, because of the greater tissue penetration of X-rays, thus addressing the issue of the shallow penetration of light, which represents a major limitation for clinical photodynamic therapy. Recently, Xie, Shen, and coworkers23 exploited this method using a novel LiGa5O8:Cr (LGO:Cr)-based nanoscintillator that emits persistent, near-infrared X-ray luminescence. LGO:Cr nanoparticles and a PS, namely, 2,3-naphthalocyanine, matching the ∼720 nm emission of the LGO:Cr nanoparticles, were encapsulated into mesoporous silica nanoparticles, to yield NC-LGO:Cr@mSiO2 nanoparticles. H1299 (human non-small cell lung carcinoma) cells underwent massive cell death upon X-ray irradiation (4 Gy) of these NC-LGO:Cr@mSiO2 nanoparticles. H1229 cells are refractory to radiotherapy and all control experiments confirmed that the cell death was due to the photodynamic effect.
The above examples illustrate some of the emerging non-conventional excitation modes that are currently applied in cancer therapies. The use of X-ray irradiation in particular for excitation of the PS may be advantageous to convince practitioners of this technique and its use in PDT, which could be feasible and desirable.
4. Tumour-microenvironment activatable PSs
As a promising strategy to address the issue of non-selective photodamage, activatable photosensitisers have received considerable attention. These smart therapeutic agents are generally quenched in the native form through various mechanisms, such as photoinduced electron transfer (PET), intramolecular charge transfer (ICT), Förster resonance energy transfer (FRET) and self-quenching, but upon interactions with tumour-associated stimuli, such as the acidic and reducing microenvironments of tumours, as well as cancer-related proteases and mRNAs, the quenching pathways are relaxed, resulting in the restoration of their fluorescence and photosensitising properties. This approach has been widely used for various classes of photosensitisers, in both molecular and nanoparticle forms.Activation under tumour acidic conditions
On the basis that tumours generally have an extracellular pH lower than normal tissues, a number of Pc-based PSs that can be activated in slightly acidic environments have been developed. These compounds usually contain amino moieties that quench the photoactivities of the Pc core through PET. Upon protonation in acidic media, this quenching pathway is inhibited, thereby restoring the fluorescence emission and singlet oxygen generation. By using a similar approach, Chen and co-workers reported a pH-responsive photosensitiser in which a ZnPc is substituted with four 2,4,6-tris(N,N-dimethylaminomethyl)phenoxy moieties, ZnPc(TAP)4 (Fig. 11).25 Both the fluorescence intensity and singlet oxygen generation efficiency of this compound were enhanced when the pH of the buffer medium was decreased from 7.4 to 6.5. The IC50 value against 4T1 mouse mammary carcinoma cells was 0.20 μM at a light dose of 2.5 J cm−2. The compound could also light up the tumour in the 4T1 tumour-bearing mice and inhibit its growth upon irradiation. Compared with the permethylated (quaternized with CH3I) analogue ZnPc(TAP)412+, ZnPc(TAP)4 showed a faster accumulation and a higher concentration in the tumour site and was more efficient in the photodynamic ablation of the tumour.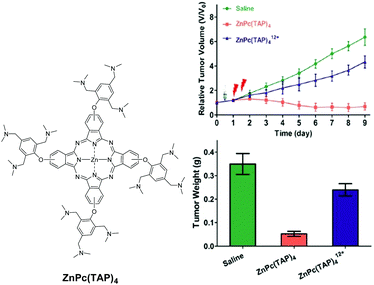 | ||
| Fig. 11 Left: Structure of the pH-responsive ZnPc(TAP)4. Right: In vivo tumour growth curves measured on 4T1 tumour-bearing mice (top), and tumour weights at the end of treatment (bottom). Adapted from ref. 25 by permission of The Royal Society of Chemistry. | ||
Apart from these molecular-based photosensitisers, a number of activatable nanophotosensitisers based on Pcs have also been reported. Huang and co-workers conjugated ZnPc molecules to stellate mesoporous silica nanoparticles (MSNs) via acid-sensitive hydrazone linkers (Fig. 12).26 As a result of self-quenching, this nanophotosensitising system remained photo-inactive in the intact form. However, both the fluorescence emission and singlet oxygen generation could be enhanced in slightly acidic solutions and inside HeLa human cervical carcinoma cells due to the cleavage of the hydrazone linkers. After intravenous injection into S180 rat ascitic tumour-bearing KM mice, this nanosystem could be activated at the tumour site and inhibit its growth with negligible systemic toxicity.
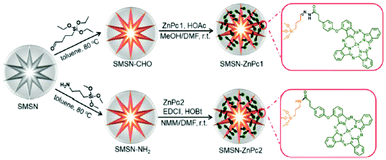 | ||
| Fig. 12 Preparation of stellate nanoparticles with pH-responsive hydrazone linkers and non-cleavable analogues. Adapted from ref. 26 by permission of The Royal Society of Chemistry. | ||
Activation under tumour redox conditions
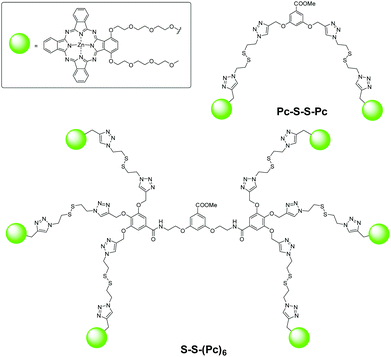
Apart from the slightly acidic environments, higher intracellular thiol concentrations, particularly glutathione (GSH), are also regarded as a biomarker for tumours. By connecting a quencher to Pcs through a thiol-cleavable linker, these compounds could become thiol-activatable, and several such examples based on the PET and self-quenching mechanisms have been reported. As an extension of the studies of self-quenched Pc dimers and trimers, a series of disulfide-linked dendritic oligomeric Pcs, from a dimer to a hexamer, were prepared using the copper-promoted alkyne–azide cycloaddition reaction as the key step.27 As expected, these compounds were significantly stacked and self-quenched in citrate solution (pH 7.4 with 1% Cremophor EL), leading to weak fluorescence intensity and low singlet oxygen generation efficiency, particularly for the higher homologues. Upon addition of GSH (5 mM), these photoactivities were promoted due to the cleavage of the disulfide linkers and separation of the Pc units. The “on/off” ratio based on the restoration of fluorescence ranged from 7 to 23. Their GSH-responsive behaviour was also demonstrated in HT29 human colon adenocarcinoma cells with IC50 values in the range of 0.18 to 0.38 μM. The biodistribution and activation of the dimer Pc-S-S-Pc and hexamer S-S-(Pc)6 (Fig. 13) in HT29 tumour-bearing nude mice were also examined. The latter seemed to have a higher enhanced permeability and retention (EPR) effect, resulting in a higher intra-tumoral fluorescence intensity over a period of 6 days.Activation by a biomarker of cancer cells
By encapsulating Pc molecules into nanoparticles, the self-quenching phenomenon renders the PSs photodynamically inactive. It provides a means for activation through stimuli-responsive disaggregation. Recently, Yoon and co-workers reported a number of these nanosystems which are formed through self-assembly of selected amphiphilic Pcs. As a recent example, they found that ZnPc with a 4-(sulfonato)phenoxy group in a non-peripheral position (PcS) could self-assemble to form a uniform nanovesicle dispersion in aqueous media, and the nanoparticles underwent disassembly upon interaction with albumin, resulting in the restoration of the fluorescence signal (Fig. 14).28 These properties enabled this nanosystem to be used for in vivo albumin labelling and tumour imaging with a relatively high signal-to-background ratio. In addition, these nanoparticles could inhibit the growth of tumours in HepG2 tumour-bearing mice upon irradiation, but the effect was much weaker in HeLa tumour-bearing mice, probably due to the faster proliferation of HeLa cells.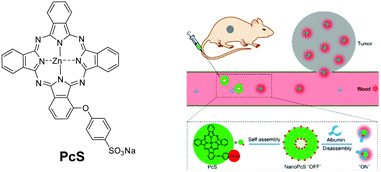 | ||
| Fig. 14 Left: Structure of PcS. Right: Self-assembled nanoparticles made of PcS and disassembly in the presence of albumin. Reproduced from ref. 28 with the permission of the American Society of Chemistry. | ||
5. Targeting strategies
Passive targeting is based on the so-called EPR (Enhanced Permeation and Retention) effect, which exploits the defective neovasculature of tumours. Systemically administrated nano-objects can exit the blood circulation system only at the tumour neovasculature defects, and hence accumulate selectively in the tumour tissues (Fig. 15). This effect is counter-balanced by the dense tumour extracellular matrix (ECM).29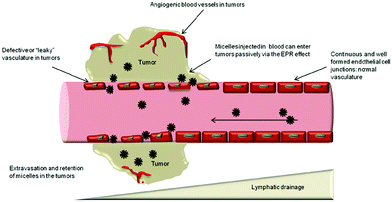 | ||
| Fig. 15 Illustration of the EPR effect. Reproduced from Frontiers (DOI: http://10.3389/fphar.2014.00077). License Creative Commons. | ||
Quite recent studies have discussed the limits of this mechanical targeting.30 Nonetheless, nanoPSs have proved to be successful in PDT.16 Their targeting efficiency is improved when actively targeting units are introduced on the nanoparticles.
Receptor/antigen targeting
In order to target the cancer-associated oncofetal Thomsen–Friedenreich disaccharide antigen (T antigen), a truncated O-glycan (Galb1-3GalNAc-O-serine/threonine) expressing in more than 90% of primary human carcinomas, but silent in healthy cells, jacalin (a lectin extracted from Jackfruit) was covalently conjugated to gold nanoparticles.31 The dimeric phthalocyanine C11Pc (Fig. 16) and a water-soluble thiol- and carboxylic acid-doubly functionalized PEG were used as stabilizers during the formation of the gold NPs to which they were covalently bound thanks to the affinity of sulphur for gold. The 155 Pc units (estimated amount) on each NP remained monomeric at their surface due to the presence of the PEG units. The jacalin was then coupled to the COOH function exposed on the outer shells of the NPs. The resulting NPs retained the SOG ability of the Pc, and were taken up into HT-29 cells through receptor-mediated endocytosis, evidencing, together with further competitive inhibition studies, the efficiency of the jacalin targeting. Compared to the non-conjugated reference compound, the jacalin-conjugated NPs exhibited outstanding phototoxicity.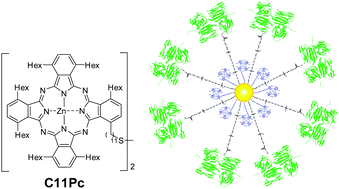 | ||
| Fig. 16 Structure of C11Pc and schematic representation of a gold NP bearing T antigen-specific jacalin and C11Pc. Adapted from ref. 31 with permission of Wiley. | ||
To overcome the tumour-protecting effect of the ECM, ZnF16Pc was encapsulated into recombined ferritin nanocages (loading rate of ∼40 wt%, ∼200 Pcs for a 12 nm overall size), the surface of the resulting construct being conjugated to a single chain viable fragment (scFv) sequence specific to fibroblast activation protein (FAP), a plasma surface protein overexpressed in nearly all cancer-associated fibroblasts (CAFs) but not in healthy fibroblasts (Fig. 17).29 The scFv-conjugated and ZnF16Pc-loaded ferritin nanoparticles (scFv-Z@FRT) aim at selectively eliminating CAFs, the main producers of the ECM fiber components, to reduce the collagen content in the ECM, and hence to lower the tumour interstitial fluid pressure for a better repartition of the NPs in the tumour, but without inducing systemic damage. Here, the photodynamic action aims at promoting NP delivery to the tumour by eliminating the obstacles between the NPs and their site of action in the tumour, rather than directly eliminating the tumour. The hydrophobic character of ZnF16Pc prevents undesired release from the ferritin nanocages. Tumour tissue binding experiments against mouse 4T1 mammary carcinoma tumour models and LL/2 Lewis lung carcinoma tumours confirmed that the scFv can target different tumours; hence, this strategy has a large potential scope of action. The tumour accumulation of 3 different types of NPs (BSA and PEGylated quantum dots (QDs) of 10 nm and 50 nm) showed their enhanced tumour uptake, 2-fold for the BSA, ∼3.4 fold for the 10 nm QDs, and up to nearly 18 fold for the 50 nm QDs.
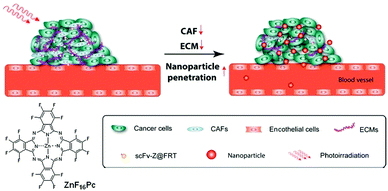 | ||
| Fig. 17 Adapted from ref. 29 with the permission of the American Society of Chemistry. | ||
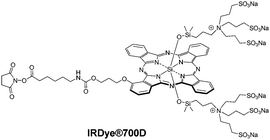
Triple-negative breast cancer (TNBC) cells do not overexpress the receptors commonly targeted by receptor-targeted therapies such as tamoxifen or trastuzumab. Photoimmunotherapy (PIT) based on the delivery of PS by antibodies is therefore a valuable alternative therapeutic option. Three single chain antibody fragments (scFv) bearing the SNAP tag and targeting receptors overexpressed in TNBC (epidermal growth factor receptor, EGFR; epithelial cell adhesion molecule, EpCAM; and chondroitin sulfate proteoglycan 4, CSPG4) have been conjugated to SiPc IR700 using the SNAP tag technology.32 To achieve the grafting by the SNAP-tag technology, IRDye®700D (Fig. 18), a commercially available phthalocyanine with a very clever design, as it is fully water-soluble, regioisomerically pure, non-aggregated and NHS-functionalized, was modified with a benzylguanine linker (BG-PEG24-NH2) to react with the scFv-SNAP-tag fusion proteins. Extremely low IC50 values (62–165 nM) for the individual or combined PSs were observed against four different TNBC cell lines (MDA-MB-468, MDA-MB-453, MDA-MB-231 and Hs758T) expressing different levels of the targeted receptors. Conjugation of IRDye®700D to a nanobody targeting G protein coupled receptor such as the extracellular part of viral GPCR US28, detected in glioblastoma, was achieved by maleimide chemistry and eradicated US28-overexpressing glioblastoma cells in two- and three-dimensional cultures.33
Photophysical and photochemical studies conducted on the IRDye®700 core (without the conjugatable fragment) showed that irradiation promoted the photolysis of the axial substituents in the presence of an electron donor, decreasing its hydrophilicity and inducing its aggregation in aqueous media and a loss of fluorescence.34IR700–monoclonal antibody (cetuximab, panitumumab or trastuzumab) conjugates exhibited the same phenomenon to a smaller extent. In vivo fluorescence experiments on an A431-luc-GFP tumour treated with cet-IR700, an MDAMB468-luc-GFP tumour treated with pan-IR700 or cet-IR700, a 3T3/Her2-luc-GFP tumour treated with tra-IR700, and a Calu3-luc-GFP tumour treated with tra-IR700 confirmed the loss of fluorescence upon NIR irradiation, associated with high cell death.
From a clinical feasibility point of view, such approaches using IR700 conjugated to antibodies are quite advanced as a Phase III clinical study on an anti-EGFR antibody–IR700-dye conjugate is currently ongoing for patients suffering from non-operable head and neck cancers (clinicaltrials.gov/ct2/show/NCT02422979).
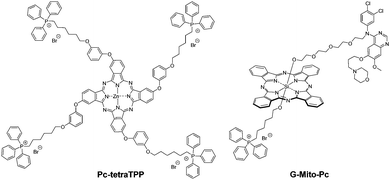
Subcellular targeting of specific organelles, in particular mitochondria, is another way to improve the photodynamic efficiency of a PS. Phthalocyanine Pc-tetraTPP (Fig. 19) was substituted with triphenylphosphonium moieties35 and proved to be an extremely powerful PS with submicromolar IC50 values against MCF-7 cells.
Phthalocyanine G-Mito-Pc (Fig. 19) has a dual targeting ability, with two targeting units in the axial positions of a SiPc.36 A gefitinib moiety targets EGFR-overexpressing cancer cells, and the triphenylphosphonium moiety in the other axial position targets the mitochondria after cellular uptake. G-Mito-Pc was tested against HeLa cells (overexpressing EGFR) and HELF cells (a normal cell line with low EGFR expression). G-Mito-Pc and a reference phthalocyanine with only a gefitinib moiety were shown to accumulate preferentially in the HeLa cells, unlike another reference phthalocyanine without any targeting unit, which accumulated the same way in both cell types. Similarly, only G-Mito-Pc which has the triphenylphosphonium moiety was shown to induce a loss of the mitochondrial membrane potential, while the other reference compounds did not. In terms of phototoxicity, 3 cancer cell lines were tested (HeLa, A549 and MDA-MB 468 cells). G-Mito-Pc proved to have a very powerful phototoxicity, with IC50 below 5 nM against the 3 types of cells, with the reference compounds being far less phototoxic.
6. Dual chemo- and photodynamic effect
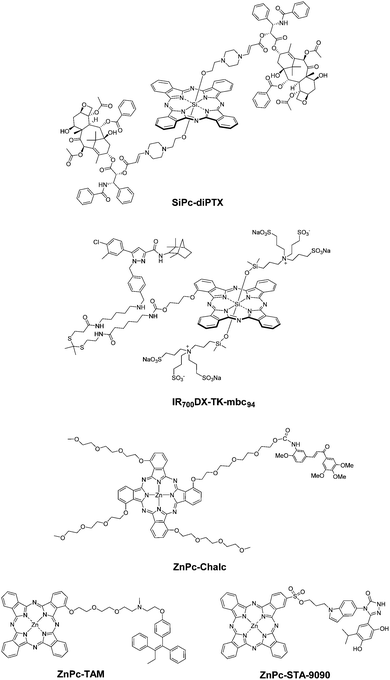
To enhance their therapeutic efficacy, it is logical to introduce additional therapeutic components into PSs so as to associate the chemotherapeutic effect with the photodynamic action. Some strategies lead to drug release from the Pc, while other combinations remain covalently linked.You and co-workers reported a SiPc with two axial paclitaxel moieties connected through singlet-oxygen-cleavable aminoacrylate linkers (SiPc-diPTX, Fig. 20).37 It was found that this prodrug was less cytotoxic and active towards tubulin polymerisation than paclitaxel. Upon irradiation, it produced singlet oxygen to kill SKOV-3 ovarian cancer cells directly and cleave the aminoacrylate linkers to release paclitaxel to induce additional cytotoxicity.
Similarly, a cannabinoid prodrug conjugated with IR700DXvia a ROS-cleavable thioketal linker was also reported (IR700DX-TK-mbc94, Fig. 20).38 This NIR light-absorbing activatable therapeutic agent could target the type-2 cannabinoid receptor CB2R overexpressed in various types of cancers with a nanomolar affinity and exert a combined photodynamic and cannabinoid therapeutic effect upon excitation at 690 nm. Its therapeutic efficacy was significantly higher than that of the analogue without the thioketal linker.
Vascular disrupting agents (VDAs), such as Combretastatin A4 and chalcones, disrupt tumour neovasculature tubules by disrupting the tubulin self-arrangement. As PDT is efficient in the oxygenated rim of the tumour, the use of VDAs is a complementary approach. A chalcone unit was linked to a water-soluble Pc via a carbamate linker to allow the release of the chalcone into biological media,39 while tetra-chalcone derivatives were too hydrophobic.40 The ZnPc-Chalc conjugate (Fig. 20) retained the SOG ability of the Pc, and likely thanks to the better amphiphilic balance exhibited better phototoxicity against HT-29 cells than the non-conjugated Pc derivative. The VDA ability of the conjugate tested against HUVECs was slightly lower than that of the non-conjugated chalcone reference derivative, due to the cleavage requirement of the conjugate. However, this approach is extremely promising.
Apart from these cleavable therapeutic agents, several non-cleavable Pc–drug conjugates have also been reported. ZnPc-TAM is conjugated with tamoxifen (Fig. 20), which can bind to estrogen receptors overexpressed in a range of breast cancer cells and is a potent drug for hormone therapy for breast cancer.41 It showed high affinity towards MCF-7 breast cancer cells and tumour tissues, causing cell death in the dark due to the cytostatic tamoxifen moiety and enhanced cytotoxicity upon irradiation due to the PDT effect of the Pc. Upon addition of exogenous 17β-estradiol as an estrogen receptor inhibitor, both the cellular uptake and photocytotoxicity of the conjugate were significantly reduced, which revealed the targeting effect of the tamoxifen moiety.
ZnPc-STA-9090 (Fig. 20) was conjugated with ganetespib (STA-9090), a heat shock protein 90 (Hsp90) inhibitor with preferential tumour selectivity.42 The ganetespib moiety promoted selective binding to extracellular Hsp90 and internalisation into tumour cells. Apart from the PDT action, this conjugate could inhibit Hsp90 activity in the tumour cells, leading to cell apoptosis.
Compared with molecular dual photodynamic and chemotherapeutic agents, the nano-counterparts have received more attention. With diverse nano-platforms, this class of advanced photosensitising systems can possess additional functionalities; hence they are described in Section 8.
From a clinical relevance and implementation point of view, the combination with drugs of existing and recognised efficiency is a positive aspect.
7. Theranostics with imaging properties
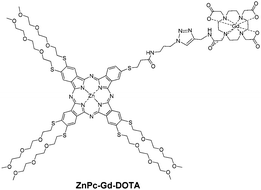
Theranostics combine therapeutic effects with imaging properties to follow the biodistribution of a PS, which is particularly useful to determine the best drug-light interval and to observe the response to the treatment. Thanks to their ability to chelate a central metal, Pcs can also act as radiosensitisers,43 and their theranostic potential has previously been acknowledged.44,45Magnetic resonance imaging is a routine imaging modality, and Gd complexes are the most widely used T1 contrast agents. Phthalocyanine ZnPc-Gd-DOTA46 (Fig. 21) was designed to be regioisomerically pure. The presence of this Gd-DOTA moiety did not affect the SOG ability of the Pc. The conjugate exhibited T1 relaxivity (r1) in the range of Omniscan® albeit slightly lower. This concept appeared to have limits as the conjugate showed no dark toxicity against MCF-7 cells, but no significant phototoxicity. Besides, due to the difference in sensitivity, as MRI needs a contrast agent in the mM concentration range, whereas photosensitising Pcs exert their photodynamic effect in the μM range, more elaborate structures, such as polymers or dendrimers, should be necessary to obtain more efficient Gd-based PDT-MRI theranostics.
Photoacoustic imaging is attracting exponential interest. Whereas nanoparticles prepared of the corresponding tetra-propargylated porphyrins proved to be good one- and two-photon PSs, silsesquioxane nanoparticles (BSPHT NPs, average size ∼ 120 nm) made from a tetra-propargylated phthalocyanine and subsequently clicked with (3-azidopropyl)-trimethoxysilane (Fig. 22) before undergoing self-condensation exhibited average photodynamic efficiency and two-photon fluorescence imaging but excellent photoacoustic properties.47 Using an analogous octapropargylated phthalocyanine gave nanoparticles with improved photodynamic properties.48
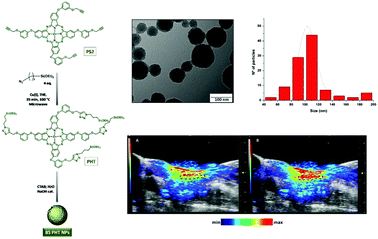 | ||
| Fig. 22 Left: Preparation of ZnPc-based silsesquioxane BSPHT NPs. Right top: TEM micrographs and size distribution. Bottom: Photoacoustic signal at 700 nm from the carotid artery (the ROI is shown with a dotted line) before and 10 seconds after intravenous injection of BSPHT NPs into the tail veins of mice. Adapted from ref. 47 by permission of The Royal Society of Chemistry. | ||
Combining Pc with graphene oxide (GO) is another means to obtain highly fluorescent PSs, with an additional photothermal effect; hence a diamino SiPc was covalently coupled to GO.49 The resulting SiPc-GO nanocomposite (Fig. 23) had an absorption extended to NIR wavelengths. Compared to pristine (unaltered) GO, SiPc-GO showed an ∼3-fold increase of absorption at wavelengths above 800 nm, with only a very slight loss of fluorescence due to energy loss, meanwhile with the retained SOG ability upon red light excitation. Albeit slightly aggregated and unlike pristine GO, the nanocomposite is well soluble in water, and proved to be internalized into HeLa cells, without dark toxicity. Once internalized, SiPc-GO exhibited good phototoxicity against HeLa and MCF-7 cells, with a predominant photodynamic effect when excited at 671 nm and a more marked photothermal effect upon excitation at 808 nm. In vivo experiments conducted on NSG female mice bearing MCF-7 tumours confirmed the fluorescence imaging ability of the composite synchronously coupled to the photodynamic and photothermal eradication of the tumours.
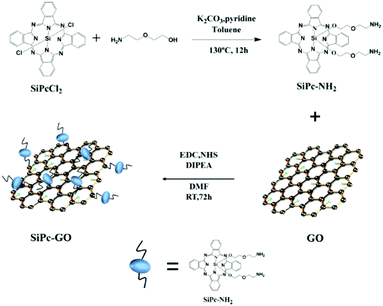 | ||
| Fig. 23 Preparation of a SiPc-GO nanocomposite. Reproduced from ref. 49 with the permission of the American Society of Chemistry. | ||
Another type of composite, made of a ZnPc covalently grafted onto TiO2 and labelled with 131I, was reported to combine PDT with nuclear imaging.50 The 131I-ZnPc-TiO2 nanocomposite was well taken up in HeLa and EMT6 tumour but less in WI-38 healthy cells, promoting a more selective photodynamic effect.
8. Combination of the different strategies
All the strategies above lead to improved photodynamic efficiencies by themselves. Combining them was obviously another way to further enhance their therapeutic success.Tumour targeting and activation
Apart from the 2,4-dinitrobenzenesulfonate (DNBS) substituent, which acts as a very efficient quencher and can be removed upon treatment with GSH, the ZnPc-DNBS-biot conjugate also contains biotin as a tumour-targeting ligand (Fig. 24).51 It showed preferential uptake by HepG2 human hepatocarcinoma cells, which have a high expression of biotin receptors, over Chinese hamster ovary (CHO) cells used as a negative control. It was also photocytotoxic (IC50 = 0.1 μM) against HepG2 cells and could localise in the endoplasmic reticulum (ER) of HepG2 cells, inducing ER stress upon irradiation.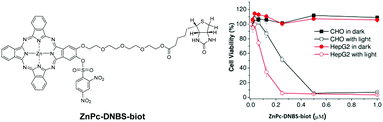 | ||
| Fig. 24 Structure of ZnPc-DNBS-biot (left) and its cytotoxic effect against HepG2 and CHO cells in the absence and presence of light (right). Adapted from ref. 51 with the permission of Wiley. | ||
Tumour activation, and dual PDT and chemotherapeutic effect
The ability of doxorubicin (DOX), a powerful anticancer chemotherapeutic, to quench SOG was exploited by linking it to a ZnPc via a tetrapeptide linker, which itself is sensitive to fibroblast activation protein (FAP), a transmembrane serine protease overexpressed in cancer-associated fibroblasts (CAFs), and capable of hydrolysing peptides having a proline in the penultimate position.52 The FAP induced release of the Pc, of which the SOG capability was restored and could exert it's a photodynamic effect, while DOX could exert a chemotherapeutic action which was partially inhibited when covalently bound to the Pc (Fig. 25).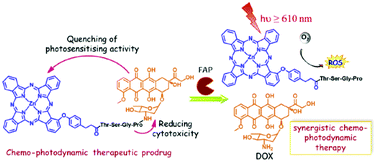 | ||
| Fig. 25 Structure of the ZnPc-TSGP-DOX conjugate and its FAP activation. Reproduced from ref. 52 with the permission of Elsevier. | ||
Tumour activation, EPR targeting, and dual PDT and chemotherapeutic effect
Lo et al. prepared a series of amphiphilic block copolymers of methoxypolyethylene glycol and poly(β-benzyl-L-aspartate) in which a ZnPc and DOX are conjugated to the aspartate side chains through an acid-labile hydrazone linker and a GSH-responsive disulfide linker respectively. These polymers could self-assemble into spherical polymeric DOX-ZnPc-micelles in aqueous media (Fig. 26).53 The release of Pc and DOX into phosphate solution and inside HepG2 cells was studied under different conditions. It was found that the micelles with a certain ratio of Pc to DOX caused a synergistic cytotoxicity, induced cell death mainly through apoptosis and showed preferential tumour localisation in tumour-bearing nude mice.53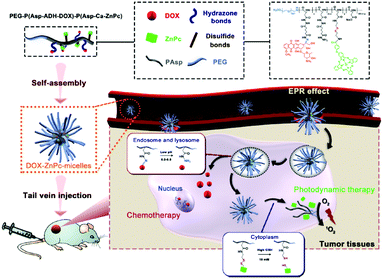 | ||
| Fig. 26 Tumour double-responsive DOX-ZnPc-micelles and their schematic mechanistic chemotherapeutic and photodynamic action. Reproduced from ref. 53 with the permission of Elsevier. | ||
DNA activation and dual photodynamic and chemotherapeutic effect
The water-soluble ZnPc tetrasubstituted with 6,8-disulfonate-2-naphthyloxy groups in non-peripheral positions, and the amphiphilic anticancer drug mitoxantrone (MA) self-assemble into uniform nanoparticles in water.54 The SOG ability of the Pc is self-quenched in these constructs in which the Pcs are highly aggregated (Fig. 27). The chemotherapeutic agent MA is meant to interact with DNA, and this interaction induces partial disruption of the nanoparticles; hence the Pc's SOG ability is recovered. In vitro (on MCF7 cells) and in vivo (on MCF7 tumour-bearing mice) experiments showed a synergistic chemotherapeutic and photodynamic effect together with a concomitant mild photothermal effect.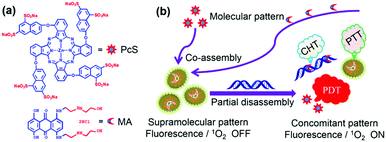 | ||
| Fig. 27 Structure of octasulphonate ZnPc and schematic mechanism of action. Reproduced from ref. 54 with the permission of the American Society of Chemistry. | ||
Imaging, tumour-targeting, and dual photodynamic and chemotherapeutic effect
Zhu and co-workers assembled a complex of unsubstituted ZnPc and soybean phosphatidylcholine, which exhibited photothermal and photoacoustic properties in the early phase, but could switch to PDT and fluorescence imaging upon disaggregation in the late phase.55 By loading DOX and further formulating the complex with 2-diacyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly(ethyleneglycol))-2000] (DSPE-PEG) and the folate-receptor-targeting DSPE-PEG-methotrexate, a multifunctional nanosystem was formed which exhibited tumour-targeting properties, switchable photoacoustic/fluorescence imaging behaviour, multiphase photothermal/photodynamic effects, and synergistic therapeutic potential.9. Conclusions and outlook
Pcs exhibit the maximum absorption in the phototherapeutic window, which makes them suitable PSs for PDT of cancer. In particular, they limit post-treatment skin sensitivity and allow the treatment of deep-seated tumours by excitation with NIR radiation. Non-conventional modes of excitation reinforce this advantage. Another advantage of Pcs is their synthetic access, often easier than other photosensitising cores absorbing in the same range of wavelengths (such as bacteriochlorins).Several phthalocyanines of the second generation of PSs are at various stages of clinical trials, and continued efforts are directed towards designing and producing Pcs with improved tumour selectivity and efficacy. Their SOG can be enhanced by heavy atoms and the importance of their amphiphilic balance is worth noting. The functionalisation at the axial positions in Pcs enhances their photophysical properties, increases their solubility and reduces their self-aggregation. Appropriate formulations (supramolecular, liposomal, etc.) have also been used to increase their biocompatibility.
Pcs belonging to the third generation of PSs are also being developed. Their photodynamic action can be combined with a known chemotherapeutic for additive or synergistic effects. Another option to increase the tumour selectivity is either to insert targeting moieties or incorporate them into targeting systems. In addition, the photoactivities of Pcs can also be activated to generate SO selectively in the tumour microenvironment as smart PSs. Finally, many combinations with imaging agents of diverse nature are reported to form theranostics to follow the biodistribution of the Pc and the effect of the treatment.
Several third-generation Pc-based PSs, as therapeutic or theranostic agents [cancer imaging (fluorescence, MR, PET) and PDT], are at different stages of preclinical investigations. The initial results obtained by using certain Pcs in combination with other therapeutic modalities may provide an effective long-term cure and survival to cancer patients. Hence, there is no doubt that Pcs are amongst the most advanced PSs for PDT of cancer.
We hope that this review showing the advantages of tetrapyrrole-based compounds in cancer-imaging and therapy, either alone or in combination with currently available therapies, will increase the awareness of the business community, and also help in receiving funds dedicated to clinical trials of the most promising agents.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
PCL thanks the Research Grants Council of the Hong Kong Special Administrative Region (Project No. 11303517) for support. RKP is thankful to NIH (PO1CA055791) and TPCI Support Grant P30CA16056. MSRM and TT have been supported by MINECO (CTQ2017-85393-P) and ERA-NET/MINECO EuroNanoMed2017-191/PCIN-2017-042. FD is grateful for the support from the Turkish Academy of Sciences.Notes and references
- W. G. Love, Photochem. Photobiol., 1996, 63, 656 CrossRef CAS PubMed.
- A. A. Brilkina, L. V. Dubasova, E. A. Sergeeva, A. J. Pospelov, N. Y. Shilyagina, N. M. Shakhova and I. V. Balalaeva, J. Photochem. Photobiol., B, 2019, 191, 128 CrossRef CAS PubMed.
- C. M. Allen, W. M. Sharman and J. E. Van Lier, J. Porphyrins Phthalocyanines, 2001, 5, 161 CrossRef CAS.
- J. Shao, J. Xue, Y. Dai, H. Liu, N. Chen, L. Jia and J. Huang, Eur. J. Cancer, 2012, 48, 2086 CrossRef CAS PubMed.
- J. D. Miller, E. D. Baron, H. Scull, A. Hsia, J. C. Berlin, T. McCormick, V. Colussi, M. E. Kenney, K. D. Cooper and N. L. Oleinick, Toxicol. Appl. Pharmacol., 2007, 224, 290 CrossRef CAS PubMed.
- M. Machacek, A. Cidlina, V. Novakova, J. Svec, E. Rudolf, M. Miletin, R. Kucera, T. Simunek and P. Zimcik, J. Med. Chem., 2015, 58, 1736 CrossRef CAS PubMed.
- F. Dumoulin, M. Durmus, V. Ahsen and T. Nyokong, Coord. Chem. Rev., 2010, 254, 2792 CrossRef CAS.
- J. Teles Ferreira, J. Pina, C. A. Fontes Ribeiro, R. Fernandes, J. P. C. Tomé, M. S. Rodríguez-Morgade and T. Torres, J. Mater. Chem. B, 2017, 5, 5862 RSC.
- J. T. Ferreira, J. Pina, C. A. F. Ribeiro, R. Fernandes, J. P. C. Tomé, M. S. Rodríguez-Morgade and T. Torres, ChemPhotoChem, 2018, 2, 640 CrossRef CAS.
- Y. Chin, S. H. Lim, Y. Zorlu, V. Ahsen, L. V. Kiew, L. Yong Chung, F. Dumoulin and H. B. Lee, PLoS One, 2014, 9, e97894 CrossRef PubMed.
- B. G. Ongarora, X. Hu, S. D. Verberne-Sutton, J. C. Garno and M. G. H. Vicente, Theranostics, 2012, 2, 850 CrossRef CAS PubMed.
- S. Makhseed, M. Machacek, W. Alfadly, A. Tuhl, M. Vinodh, T. Simunek, V. Novakova, P. Kubat, E. Rudolf and P. Zimcik, Chem. Commun., 2013, 49, 11149 RSC.
- L. M. O. Lourenço, P. M. R. Pereira, E. Maciel, M. Válega, F. M. J. Domingues, M. R. M. Domingues, M. G. P. M. S. Neves, J. A. S. Cavaleiro, R. Fernandes and J. P. C. Tomé, Chem. Commun., 2014, 50, 8363 RSC.
- S. Ghosh, K. A. Carter and J. F. Lovell, Biomaterials, 2019, 218, 119341 CrossRef CAS PubMed.
- C. F. van Nostrum, Adv. Drug Delivery Rev., 2004, 56, 9 CrossRef CAS PubMed.
- L. V. Kiew, H. Y. Cheaha, S. H. Voon, E. Gallon, J. Movellan, K. H. Ng, S. Alpugand, H. B. Lee, F. Dumoulin, M. J. Vicent and L. Y. Chung, Nanomedicine, 2017, 13, 1447 CrossRef CAS PubMed.
- H. Y. Cheah, E. Gallon, F. Dumoulin, S. Z. Hoe, N. Japundžić-Žigon, S. Glumac, H. B. Lee, P. Anand, L. Y. Chung, M. J. Vicent and L. V. Kiew, Mol. Pharmaceutics, 2018, 15, 2594 CrossRef CAS PubMed.
- B. Pucelik, I. Gürol, V. Ahsen, F. Dumoulin and J. M. Dabrowski, Eur. J. Med. Chem., 2016, 124, 284 CrossRef CAS PubMed.
- J. Voskuhl, U. Kauscher, M. Gruener, H. Frisch, B. Wibbeling, C. A. Strassert and B. J. Ravoo, Soft Matter, 2013, 9, 2453 RSC.
- J. Tan, J. Meeprasert, Y. Ding, S. Namuangruk, X. Ding, C. Wang and J. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 40132 CrossRef CAS PubMed.
- U. Kostiv, V. Patsula, A. Noculak, A. Podhorodecki, D. Větvička, P. Poučková, Z. Sedláková and D. Horák, ChemMedChem, 2017, 12, 2066 CrossRef CAS PubMed.
- R. Abdurahman, C.-X. Yang and X.-P. Yan, Chem. Commun., 2016, 52, 13303 RSC.
- H. Chen, X. Sun, G. D. Wang, K. Nagata, Z. Hao, A. Wang, Z. Li, J. Xie and B. Shen, Mater. Horiz., 2017, 4, 1092 RSC.
- L. Larue, A. Ben Mihoub, Z. Youssef, L. Colombeau, S. Acherar, J. C. André, P. Arnoux, F. Baros, M. Vermandel and C. Frochot, Photochem. Photobiol. Sci., 2018, 17, 1612 RSC.
- S. Yan, J. Chen, L. Cai, P. Xu, Y. Zhang, S. Li, P. Hu, X. Chen, M. Huang and Z. Chen, J. Mater. Chem. B, 2018, 6, 6080 RSC.
- A.-L. Lin, S.-Z. Li, C.-H. Xu, X.-S. Li, B.-Y. Zheng, J.-J. Gu, M.-R. Ke and J.-D. Huang, Biomater. Sci., 2019, 7, 211 RSC.
- S. Y. S. Chow, R. C. H. Wong, S. Zhao, P.-C. Lo and D. K. P. Ng, Chem. – Eur. J., 2018, 24, 5779 CrossRef CAS PubMed.
- X. Li, S. Yu, Y. L. T. Guo, N. Kwon, D. Lee, S. C. Yeom, Y. Cho, G. Kim, J.-D. Huang, S. Choi, K. T. Nam and J. Yoon, J. Am. Chem. Soc., 2019, 141, 1366 CrossRef CAS PubMed.
- L. Li, S. Zhou, N. N. Lv, Z. Zhen, T. Liu, S. Gao, J. Xie and Q. Ma, Mol. Pharmaceutics, 2018, 15, 3595 CrossRef CAS PubMed.
- M. Torrice, ACS Cent. Sci., 2016, 2, 434 CrossRef CAS PubMed.
- G. Obaid, I. Chambrier, M. J. Cook and D. A. Russell, Angew. Chem., Int. Ed., 2012, 51, 6158 CrossRef CAS PubMed.
- M. Amoury, D. Bauerschlag, F. Zeppernick, V. von Felbert, N. Berges, S. Di Fiore, I. Mintert, A. Bleilevens, N. Maass, K. Bräutigam, I. Meinhold-Heerlein, E. Stickeler, S. Barth, R. Fischer and A. F. Hussain, Oncotarget, 2016, 7, 54925 CrossRef PubMed.
- T. W. M. De Groof, V. Mashayekhi, T. S. Fan, N. D. Bergkamp, J. Sastre Toraño, J. R. van Senten, R. Heukers, M. J. Smit and S. Oliveira, Mol. Pharmaceutics, 2019, 16, 3145 CrossRef CAS PubMed.
- K. Sato, K. Ando, S. Okuyama, S. Moriguchi, T. Ogura, S. Totoki, H. Hanaoka, T. Nagaya, R. Kokawa, H. Takakura, M. Nishimura, Y. Hasegawa, P. L. Choyke, M. Ogawa and H. Kobayashi, ACS Cent. Sci., 2018, 4, 1559 CrossRef CAS PubMed.
- M. Tarhouni, D. Durand, E. Önal, D. Aggad, Ü. İşci, G. Ekineker, F. Brégier, B. Jamoussi, V. Sol, M. Gary-Bobo and F. Dumoulin, J. Porphyrins phthalocyanines, 2018, 22, 552 CrossRef CAS.
- X. Zhao, Y. Huang, G. Yuan, K. Zuo, Y. Huang, J. Chen, J. Li and J. Xue, Chem. Commun., 2019, 55, 866 RSC.
- P. Thapa, M. Li, M. Bio, P. Rajaputra, G. Nkepang, Y. Sun, S. Woo and Y. You, J. Med. Chem., 2016, 59, 3204 CrossRef CAS PubMed.
- X. Ling, S. Zhang, Y. Liu and M. Bai, J. Biomed. Opt., 2018, 23, 108001 Search PubMed.
- S. Tuncel, A. Trivella, D. Atilla, K. Bennis, H. Savoie, F. Albrieux, L. Delort, H. Billard, V. Dubois, V. Ahsen, F. Caldefie-Chézet, C. Richard, R. W. Boyle, S. Ducki and F. Dumoulin, Mol. Pharmaceutics, 2013, 10, 3706 CrossRef CAS PubMed.
- S. Tuncel, J. Fournier-dit-Chabert, F. Albrieux, V. Ahsen, S. Ducki and F. Dumoulin, Org. Biomol. Chem., 2012, 10, 1154 RSC.
- F.-L. Zhang, M.-R. Song, G.-K. Yuan, H.-N. Ye, Y. Tian, M.-D. Huang, J.-P. Xue, Z.-H. Zhang and J.-Y. Liu, J. Med. Chem., 2017, 60, 6693 CrossRef CAS PubMed.
- L. Huang, G. Wei, X. Sun, Y. Jiang, Z. Huang, Y. Huang, Y. Shen, X. Xu, Y. Liao and C. Zhao, Eur. J. Med. Chem., 2018, 151, 294 CrossRef CAS PubMed.
- H. Ali and J. E. van Lier, Chem. Rev., 1999, 99, 2379 CrossRef CAS PubMed.
- L. B. Josefsen and R. W. Boyle, Theranostics, 2012, 2, 916 CrossRef CAS PubMed.
- Y. Zhang and J. F. Lovell, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9, e1420 Search PubMed.
- D. Aydın Tekdas, R. Garifullin, B. Şentürk, Y. Zorlu, U. Gundogdu, E. Atalar, A. B. Tekinay, A. A. Chernonosov, Y. Yerli, F. Dumoulin, M. O. Guler, V. Ahsen and A. G. Gürek, Photochem. Photobiol., 2014, 90, 1376 CrossRef PubMed.
- C. Mauriello-Jimenez, M. Henry, D. Aggad, L. Raehm, X. Cattoën, M. W. C. Man, C. Charnay, S. Alpugan, V. Ahsen, D. K. Tarakcı, P. Maillard, M. Maynadier, M. Garcia, F. Dumoulin, M. Gary-Bobo, J.-L. Coll, V. Josserand and J.-O. Durand, Nanoscale, 2017, 9, 16622 RSC.
- G. Ekineker, C. Nguyen, S. Bayır, S. Dominguez Gil, Ü. İşci, M. Daurat, A. Godefroy, L. Raehm, C. Charnay, E. Oliviero, V. Ahsen, M. Gary-Bobo, J.-O. Durand and F. Dumoulin, Chem. Commun., 2019, 55, 11619 RSC.
- J. Pan, Y. Yang, W. Fang, W. Liu, K. Le, D. Xu and X. Li, ACS Appl. Nano Mater., 2018, 1, 2785 CrossRef CAS.
- F. Yurt, K. Ocakoglu, M. Ince, S. G. Colak, O. Er, H. M. Soylu, C. Gunduz, C. B. Avci and C. Caliskan Kurt, Chem. Biol. Drug Des., 2018, 91, 789 CrossRef CAS PubMed.
- L. Yu, Q. Wang, K.-W. Yeung, W.-P. Fong and P.-C. Lo, Chem. – Asian J., 2018, 13, 3509 CrossRef CAS PubMed.
- M.-R. Ke, S.-F. Chen, X.-H. Peng, Q.-F. Zheng, B.-Y. Zheng, C.-K. Yeh and J.-D. Huang, Eur. J. Med. Chem., 2017, 127, 200 CrossRef CAS PubMed.
- D. Gao and P.-C. Lo, J. Controlled Release, 2018, 282, 46 CrossRef CAS PubMed.
- X. Li, S. Yu, D. Lee, G. Kim, B. Lee, Y. Cho, B.-Y. Zheng, M.-R. Ke, J.-D. Huang, K. T. Nam, X. Chen and J. Yoon, ACS Nano, 2018, 12, 681 CrossRef CAS PubMed.
- J. Ma, D. Chen, Y. Li, Y. Chen, Q. Liu, X. Zhou, K. Qian, Z. Li, H. Ruan, Z. Hou and X. Zhu, J. Controlled Release, 2018, 284, 1 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |