Open Access Article
Open Access ArticlePoly(ionic liquid) composites†
Su-Yun
Zhang‡
 ab,
Qiang
Zhuang‡
c,
Miao
Zhang
d,
Hong
Wang
ab,
Qiang
Zhuang‡
c,
Miao
Zhang
d,
Hong
Wang
 e,
Zhiming
Gao
a,
Jian-Ke
Sun
*a and
Jiayin
Yuan
e,
Zhiming
Gao
a,
Jian-Ke
Sun
*a and
Jiayin
Yuan
 *d
*d
aSchool of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, P. R. China. E-mail: jiankesun@bit.edu.cn
bCollege of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060, P. R. China
cDepartment of Applied Chemistry, School of Science, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, P. R. China
dDepartment of Materials and Environmental Chemistry, Stockholm University, 10691 Stockholm, Sweden. E-mail: jiayin.yuan@mmk.su.se
eKey Laboratory of Functional Polymer Materials (Ministry of Education), Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin, 300071, P. R. China
First published on 25th February 2020
Abstract
Poly(ionic liquid)s (PILs), as an innovative class of polyelectrolytes, are composed of polymeric backbones with IL species in each repeating unit. The combined merits of the polymers and ILs make them promising materials for composites in materials science. Particularly, the integration of PILs with functional substances (PIL composites) opens up a new dimension in utilizing ionic polymers by offering novel properties and improved functions, which impacts multiple subfields of our chemical society. This review summarizes recent developments of PIL composites with a special emphasis on the preparation techniques that are based on the intrinsic properties of the PILs and the synergistic effects between the PILs and substances of interest for diverse applications.
1. Introduction
Poly(ionic liquid)s (PILs), as the polymerization products of an ionic liquid (IL) monomer, have emerged in the last decade as an interdisciplinary topic across multiple research fields, i.e., polymers, ILs and materials science; the wide interest in them is due to their broad range of physicochemical properties and their abundance of chemical structure designs with the use of various IL monomers and backbones.1–5 Owing to the IL species in its repeating unit, a PIL may exhibit precisely tunable physical and chemical properties, i.e., solubility, ion conductivity (up to 10−3 S cm−1), electrochemical stability (up to 5 V vs. Li+/Li0), thermal stability (300 °C or even higher), and nonflammability, depending on the type of cation–anion pair, a behavior that is similar to ILs. To integrate their advanced property profile into a variety of applications, PILs can be tailored to compatibly interact with materials of interest through the IL and modern polymer chemistry.6–10 Reviews of PILs and their material applications have been published by several pioneering groups,1–6 and they have described a diverse range of topics, including a systematic study of the synthetic strategies, an analysis of the extent of PIL chemical structures, an analysis of physical properties, and a review of their material applications as well as biorelated topics. For example, the synthetic aspects of PILs and the main aspects related to their physicochemical properties (solution and solid-state properties) were reviewed by Mecerreyes in 2011.4 Our group offered a comparative review of PILs, including synthesis and applications in thermoresponsive materials, carbon materials, and energy harvesting/generation in 2013.2 In 2017, Yan and Texter et al. timely highlighted and updated the novel material applications of PILs over the past few years.5Currently, PILs have dramatically changed the research scope of traditional ionic polymers and polyelectrolytes. This statement can be especially evidenced in terms of their interaction and compatibility with materials actively studied in other fields, such as inorganic materials, metal–organic hybrids and (bio)macromolecules. Controlled integration of PILs and substances of interest may create advanced functional (nano)composites exhibiting intriguing properties that are absent in or are superior to individual components. Polymer materials have long intrigued many scientific communities, and PIL composite materials are opening up a new dimension in utilizing ionic polymers to offer unique properties and improved functions. To date, PILs have successfully formed many functional composites with metal salts or metal nanostructures, polyoxometalates (POMs), silicas, organic compounds, polymers, metal–organic frameworks (MOFs), carbons (amorphous carbons, graphene, carbon nanotubes), biomaterials, and more.11–20 Such composites are intensively explored for use in sorption, separation and catalysis processes and in products including batteries, supercapacitors, fuel cells, photovoltaic devices, sensing platforms, and so on.21–25
This review will expound this research trend and promote efforts to understand the fundamentals of the role of PILs as an active component for composite design and applications. Furthermore, this review places a particular emphasis on comprehending how to engineer and prepare multifunctional composite materials with advanced functions. In our opinion, this review will inspire and catalyze new research directions to fuel the growth of the PIL material family into a wide range.
2. Composite-related properties of PILs
2.1 Structural diversity
The properties of PILs can be varied by tuning the structure of the ion-pair composition and/or the polymer backbone. Even when produced from the same IL monomer, PILs can be different when adopting a variety of polymer chain architectures, such as linear, star-shaped, (hyper)branched and cyclic. In addition, an IL monomer can be covalently linked to neutral monomers to form a random, block or grafting copolymer. Given the tremendous array of polymer types, there are unprecedented options in copolymer design. The IL monomer may randomly copolymerize with a common nonionic monomer to dilute the charge character and therefore form a PIL copolymer with an adjustable charge density. In principle, PILs prepared from mechanisms other than traditional (co)polymerization techniques (e.g., free radical polymerization, step-growth polymerization and ring-opening polymerization) can further enrich their structural toolbox. Such diversity in macromolecular design makes the PIL family adaptive to a broad range of composite fabrications.2.2 Adaptive solubility
PIL structures and properties can be modulated readily and uniquely by counterion metathesis, which is experimentally much simpler than restructuring their backbone. In particular, PILs can be shuttled between a hydrophilic and a hydrophobic state through counterion exchange reactions with a foreign salt. For example, by adding a bis(trifluoromethane sulfonyl)imide (CF3SO2)2N−, abbreviated as TFSI− or hexafluorophosphate (PF6−)-containing salt into an aqueous solution of a halide-bearing PIL, a thermodynamically driven anion exchange occurs. These anion metathesis reactions allow the PIL to carry TFSI− or PF6− as the counter anion and become hydrophobic enough to precipitate out of its aqueous solution. After separation, it can be dissolved in a targeted organic solvent that may not dissolve the original halide-containing PIL.26 Such tunable solubility enables a wide scope of processing conditions when crafting composites in polar or nonpolar solvents. A soluble or dispersible PIL in a liquid can electrostatically interact with charged species to associate the PIL with ions, molecules, complexes, polymers, or charged surfaces of insoluble materials, thus laying a foundation for forming composites. These solution mixture systems are compatible with some classical polymer processing methods, such as drop casting, spin coating, electrospinning, and extrusion. Thus, PIL-based composites with well-defined morphologies, such as thin films and long fibers, can be accessed, a feature that is important for specific material applications where distinctive, stable shapes are popular for fabrication and assembly with other components into functional devices.2.3 Chemical stability
Chemical stability is a prerequisite for practical usage. It has been recognized that due to the well-known high stability of IL moieties, PILs can be argued to be more resistant to chemical degradation than most neutral polymers. Nevertheless, side reactions such as structural rearrangement or crosslinking might unexpectedly occur under complex chemical circumstances in a particular device or reaction. For example, the hydroxide anion in PILs might give rise to chemical instability at a high concentration because of its high nucleophilicity.27 In this context, PILs carrying imidazolium cations might undergo a ring-opening reaction under strongly alkaline conditions at an elevated temperature. It is common knowledge that the C2-proton in an imidazolium ring or the C3-/C5-protons in a 1,2,4-triazolium ring are active under basic conditions, forming active (poly)carbene intermediates to further react with other substances.282.4 Thermal stability
Thermal stability defines the operation and processing temperature range of the PIL composites. It is known that the thermal stability of polymers depends on both the repeating units and the backbone. In PILs, the ion pair of the IL unit seems more dominant in defining their thermal stability.29 Experimentally, PILs depending on the chemical structures of ion pairs and backbones show a wide range of decomposition onset temperatures (Tonset) that are between 150 and 400 °C.30 In the PIL Tonset database, it is revealed that similar to neutral polymers, the presence of aromatic groups in PIL structures rather than aliphatic groups promotes thermal stability. The thermal stability of PILs decreases with an increasing alkyl spacer length that connects the polymer backbone and the ion-pair component. In terms of cation–anion pairs, the thermal stability of PILs follows the general sequence of imidazolium ∼ pyridinium > pyrrolidinium > ammonium for cations and [Br]− < [PF6]− < [BF4]− < [CF3SO3]− < [TFSI]− for anions.31 Elabd and coworkers investigated the effect of anions on the thermal stability of poly(1-[(2-methacryloyloxy)ethyl]-3-butylimidazolium X) (X ∼ anion) and found that the variations in decomposition temperatures for PILs reached ∼45 °C. Similarly, Mecerreyes and coworkers tested PILs derived from poly(1-ethyl-3-vinylimidazolium bromide), poly(1-ethyl-4-vinylpyridinium bromide), and poly(methacryloyloxyethyltrimethylammonium chloride). The thermal stability was identified to be in the order of [CF3SO3]− > [TFSI]− > [C12H25C6H4SO3]− > [PF6]− > [Br]− > [C16H34PO4]−.322.5 Tunable ion conductivity
Ion conductivity is a fundamental property of PILs due to their ionic nature. As candidate materials for solid-state electrolytes, PILs attract a broad interest for battery- and fuel cell-related applications. In some cases, a PIL combined with other conductive species could drastically enhance the performance in electrochemical devices.33 Therefore, understanding PIL ion conductivity is a prerequisite for designing advanced composites for energy devices. After examining the structure of PILs, it is observed that the ion conductivity arises from the motion of counter ions affiliated with the polymer backbone in an electrical field. As most reported PILs are in a solid-state near room temperature, only counter ions behave as charge carriers, while the motion of the ionic backbone is negligible. In other words, PILs are quasi-single-ion conductors. This effect is responsible for PILs having a lower conductivity than their monomeric ILs. For example, the ion conductivity decreased from ∼10−2 to 10−6 S cm−1 for 1-ethyl-3-vinylimidazolium TFSI− after polymerization.34 However, structural diversity in PILs enables tunable ion conductivity. As summarized from recent literature, the ion conductivity of PILs depends on chemical structures, including the backbone, ion pairs,35 and the spacer between the backbone and ion pairs.34,36 Elabd and coworkers reported that the ion conductivity increased by over an order of magnitude in copolymers built up from a hexyl methacrylate (HMA) and a methacrylate-based imidazolium IL when the content of the nonionic HMA was increased; that is, the decreased concentration of charge carriers, i.e., the IL density in copolymers, was overcompensated by the lowered glass transition temperature (Tg), thus providing increased ion mobility.37 Bearing a low Tg of −14 °C, poly(1-glycidyl-3-butylimidazolium TFSI) achieved a high ion conductivity of 10−5 S cm−1 at 30 °C.38 By varying the spacer length between the polymerizable group (the backbone) and the distal imidazolium ring, the ion conductivity can reach 1.37 × 10−4 S cm−1 at 30 °C.39 In addition, analogous to hydrophobic ILs, a trace amount of water is readily absorbed into PILs, which facilitates ion transport; this is due to the high-sensitivity of ion conductivity toward the moisture content of the external environment.40 Such attributes in combination with functional substances may be desirable when fabricating composites for sensors and soft actuators.2.6 Broad electrochemical stability window
PILs bear a part of, if not all, the merits of ILs. Thus, PILs are nonflammable, nonvolatile, and ion-conductive, and they exhibit a wide electrochemical window ranging up to 5.0 V vs. Li+/Li0. This allows PILs to be used as electrolytes for electrochemical operations that are difficult for molecular solvent/electrolyte systems. For pyrrolidinium PILs with TFSI− as the counter anion, the electrochemical window was reported to be up to 5.0 V vs. Li+/Li0, and this value decreased at high temperatures.41 Depending on the polymerized ions and semi-free counterions, the electrochemical windows of PILs may be subtly tuned. As mentioned above, the properties of PILs are readily adjustable by the nature of the counterions. Thus, the oxidative-limit sequence of anions, i.e., [TFSI]− > [tris(pentafluoroethyl)trifluorophosphate]− > [TfO]− > [dicyanamide]− > [TFA]−,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 for ILs can be a reference for designing electrochemically stable PILs.43 In addition, a recent study on cations in PILs showed that generally speaking, pyrrolidinium-based PILs currently exhibit the widest electrochemical window.44
42 for ILs can be a reference for designing electrochemically stable PILs.43 In addition, a recent study on cations in PILs showed that generally speaking, pyrrolidinium-based PILs currently exhibit the widest electrochemical window.44
2.7 Universal compatibility
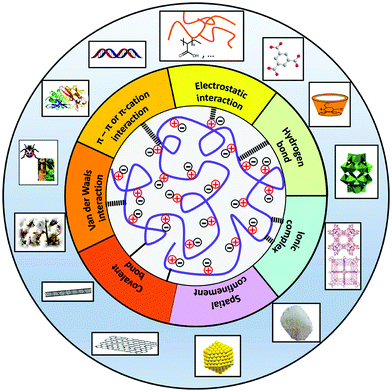
PILs are surface-active materials and are compatible with many materials, including inorganics, organics, inorganic–organic hybrids, and biomaterials. The wide compatibility of PILs toward different substances is ascribed to the noncovalent and covalent bonding categories according to the nature of interactions.Noncovalent interaction-based hybrids can be assembled through electrostatic interactions, cation–π interactions, hydrogen bonding, and van der Waals interactions. For example, a poly(1-vinyl-3-ethylimidazolium bromide) PIL can transfer CNTs from an aqueous to an organic phase by an anion exchange reaction of [Br−] with [TFSI−]45 because of the “cation–π” attractive interaction between the imidazolium cation and the CNT surface.46,47 Similarly, they can stabilize graphene, which contains π-conjugated aromatic rings,48,49 and switch the surface properties of the graphene sheets from a hydrophilic to a hydrophobic phase by an anion exchange reaction.50 In the area of developing electrodes for high-performance supercapacitors, PILs attached to the surface of graphene oxide (GO) enhanced the compatibility of GO with the IL electrolyte to enlarge the effective electrode surface area that was accessible to electrolyte ions; as such, the electrode reached a maximum energy density of 6.5 W h kg−1 with a power density of 2.4 kW kg−1.51 The ability to utilize electrostatic interactions is another aspect that can enhance the compatibility of PILs with other substances. By using ion metathesis reactions, functional counterions such as POMs can be introduced into PIL systems. Such electrostatic interactions can even create hybrid materials bearing nanoporous PIL–acid complexes.52 In addition to electrostatic interactions, PILs also show hydrogen bonding, for example with proton donors such as amino acids.53
Covalent binding between PILs and substances is an alternative for composite fabrication. For example, PILs with abundant coordination sites (N, O and P) in their heterocyclic ring can covalently bind to metal substances via coordination bonds, leading to the formation of stable composites. Such compatibility with different metal species enables control over the size of nanostructured materials and their morphology.54,55 In addition, chemical reactions can covalently attach a PIL to different substances. For example, surface-initiated atom transfer radical polymerization (ATRP) enabled a PIL to grow onto a silicon wafer for functional textiles and intelligent microfluidic switching.56 It is worth noting that covalent hybrid PILs have been generally less studied than noncovalent PIL composites and have high potential for further integration/functionalization; also the noncovalent PIL composites provide a platform for the construction of interesting supramolecular assemblies.57
3. Synthetic methods
To date, huge efforts have been directed to synthesize PIL-based composites, aiming not only to improve the compatibility of PILs with other components but also to take advantage of the multifunctionality and enhanced properties of PILs for better materials. These composites are widely used in applications of solid-state electrolytes, separation membranes, selective catalysts, sorbents, electrochemical sensors, supercapacitors, and liquid chromatography. As mentioned above, to synergistically combine substances of interest with PILs, the interactions need to be carefully chosen based on chemical structures and physical properties. Typically, the compatible interactions so far reported in the literature of PIL composites can be summarized into two kinds, namely, class I (covalent) and class II (noncovalent), which we have mentioned in Section 2.7. In this review, we classify the fabrication of PIL composites into one-step and multistep methods. Table 1 summarizes the advantages and disadvantages of the typical synthetic methods for PIL composites (Scheme 1).| Methodology | Advantages | Disadvantages | |
|---|---|---|---|
| One-step | Solid–solution mixing | Direct and easy to perform | Difficult to generate a uniform composite structure |
| Solution–solution mixing | Easy to perform; a structurally uniform composite; the mixture solution can be further processed into a device, e.g., thin film | Limited to substances that are soluble in solvents | |
| Multistep | In situ polymerization of an IL monomer in IL-substances | Composites are designable by a judicious choice of IL monomers and polymerization methods | The synthetic procedure is relatively complicated |
| In situ conversion of a substance precursor bound to a PIL | Tailor-made; the (size/spatial) distribution of substances in the composite is well controlled | Complicated synthesis; the PIL should be chemically robust in reaction media during the precursor transformation | |
3.1 One-step method
The one-step method is to directly mix a PIL solution with pre-synthesized or commercial substances, including carbons, metal salts, nanocrystals, and organics, via covalent/noncovalent interactions. This method involves two approaches: solid–solution mixing and solution–solution mixing.Alternatively, PILs in a crosslinked and insoluble powdered form can be mixed with solutions of substances to prepare composites. This procedure provides good opportunities for importing PIL functionality into the new composites. Dai and coworkers used a series of crosslinked PIL networks (six ion pairs per IL monomer unit) hosting LiTFSI in a solution as quasi-solid electrolytes in lithium-metal batteries. The solid-like polymer electrolytes exhibited an ion conductivity at room temperature of up to 5.3 × 10−3 S cm−1 by varying the ion density in the PIL network. Such crosslinked PILs provide abundant, weakly coordinating sites for Li+ transport via electrostatic interactions. Moreover, their hierarchical and robust backbone provides the mechanical strength needed in the electrolyte membrane as well as a rich free volume for Li+ motion.8 In another report, Yan and coworkers doped transition metal salts (CuCl2, FeCl3 and ZnCl2) into a photocrosslinked imidazolium PIL powder via coordination interactions to enhance antibacterial activities. The presence of metal ions generated reactive oxygen species (ROS), such as singlet oxygen (1O2), that progressively oxidized the bacterial cell wall.60 Yang and coworkers immobilized Cr3+ ions onto a SO3H-functionalized solid PIL via coordination interactions. Simultaneous functionalization with Brønsted/Lewis acidic groups in such heterogeneous catalysts enhanced catalytic activity.61
The solid–solution mixing approach features a facile and feasible way to import the functions of PILs into their composites, such as enhanced dispersibility, ion conductivity, and thermal stability. Nevertheless, only limited examples have been reported so far. In most cases, such heterogeneous solid–solution mixing cannot efficiently generate uniform composites, which may compromise the performance of the fabricated products to some extent.
PILs in solution are solvated by solvent molecules and thus are more weakly paired with counterions than when they are in their solid-state. Consequently, it is feasible to incorporate other anionic species into the polymeric skeletons by counterion metathesis reactions. For example, a variety of PIL–POM composites can be prepared by mixing a cationic PIL and an anionic POM aqueous solution due to Coulombic attraction between them. Enhanced photosensitivity in a PIL–POM composite was achieved for use in photocatalysis.62 More examples of PIL–POM composites are discussed in Section 4.5.
In addition to using ion metathesis, electrostatic interaction is also an effective strategy to produce new functional PIL composites. Our group reported an interpolyelectrolyte complexation method between a cationic hydrophobic PIL and an organic multiacid/polyacid by electrostatic crosslinking in organic solvents.63 The obtained materials exhibited a nanoporous structure useful in catalysis, sensing and gas sorption.64 In the long history of polyelectrolyte materials, interpolyelectrolyte complexation in an aqueous phase is dominant. Nevertheless, in the absence of an external inorganic template, such as silica nanoparticles, various attempts to synthesize interpolyelectrolyte complexes have resulted in nonporous or products with pore size far beyond 100 nm. Due to their adaptive solubility, PILs expand the scope of polyelectrolyte complexation from an aqueous to an organic medium. The difference in ionic complexation in water and organic solvents is that the hydrophobic interaction basically diminishes in organic media, and hydrogen bonding can be weakened or canceled as well. Both of them are crucial in shaping the microstructure of the complex products in addition to the Coulombic interaction.
Additionally, weak interactions such as hydrogen bonding and host–guest complexation increase the tools to engineer PIL composites in the solution–solution mixing approach. Charlot and coworkers employed an imidazolium-based IL as the solvent to dissolve guar gum and poly-(1-[2-acryloylethyl]-3-methylimidazolium bromide), which formed a highly conductive (7.5 × 10−4 S cm−1 at 30 °C) and elastic ionogel (elastic modulus up to 30 kPa) via hydrogen bonds and topologic chain entanglements. The formation of uniform guar/PIL associations in the IL medium enabled a good compromise between the mechanical cohesion and the ion mobility.65 Ritter and coworkers prepared PIL–organic molecule composites by using cyclodextrin (CD) and poly(1-vinyl-3-butylimidazoloum TFSI).66 The PIL–CD complex showed pseudo-LCST (lower critical solution temperature) behavior in solution due to the reversible guest–host complexation between CD and TFSI−.
Coordination interactions derived from the functional coordinating sites (N, O, S, and P) in the PIL backbone can drive composite formation in solution, especially for metal or metal complexes. CdSe nanocrystals can coordinate with an imidazolium PIL poly(1-vinyl-3-butylimidazolium PF6) or a PIL-functionalized homolog with a thiol end-group by simply mixing in DMSO.67 Charge transfer processes may occur between the two components to facilitate the potential integration of PIL into photovoltaic and optoelectronic devices.
In the solution–solution mixing approach, both the PIL and other substances undergo Brownian motion accompanied by a variety of other interactions to drive the formation of the final composites. In this case, PIL composites bearing these interactions can synergistically work with each other, leading to better materials. For example, a PIL-based gel polymer electrolyte was prepared by mixing N-ethyl-N-methylpyrrolidinium TFSI (P12FSI) and a pyrrolidinium-based PIL and LiTFSI in acetone, which was drop-cast into a membrane.68 The charged PIL macromolecular structure was associated with ILs and Li+via electrostatic and coordination interactions. This membrane composite was flexible and thermally and electrochemically stable and carried a desirable room temperature ionic conductivity and good interfacial stability with the lithium metal. Schönhoff and coworkers used the same strategy to mix poly(N,N-diallyl-N,N-dimethylammonium X) (PDADMA-X, X denotes the anion type) with a Li salt and ILs in DMF to form a gel polymer electrolyte69 that had improved compatibility and was free of leakage under pressure/vacuum conditions. Similarly, a PIL/IL–Ag composite membrane was obtained by casting a mixture solution of poly(1-butyl-1-methylpyrrolidinium TFSI) (poly[pyr14][TFSI]) and AgTFSI, which enhanced the permeability of both C2H4 and C2H6 and overcame the hindered gas diffusion in the pristine PIL.70 The silver salt in the composites boosted the solubility of olefin in the membranes and, upon optimization, enhanced the overall C2H4/C2H6 permselectivity.
3.2 Multistep method
A multistep method involves binding the precursor of a substance (substance) to a PIL (IL monomer unit), followed by a chemical reaction to generate PIL composites. In this approach, tailor-made composite structures based on delicate synthetic chemistry can be rationally designed to produce composites with controlled functions and properties.Polymerization of an IL monomer contained inside hosts is effective to construct PIL composites with prominent synergetic properties due to the possibility of nanoconfinement. For example, Jiang and coworkers integrated imidazolium PILs into the MIL-101 MOF via in situ polymerization of encapsulated monomers. The synergistic effects between the good CO2 enrichment and the Lewis acid sites in the PIL and MOF are responsible for the high activity of these PIL composites toward a cycloaddition reaction of CO2 with epoxides; the reaction can form cyclic carbonates at sub-atmospheric pressure. Li and coworkers synthesized a PIL–MOF composite by threading PIL into the UiO-66 MOF via in situ polymerization, which exhibited excellent selectivity and fast kinetics for ion exchange, demonstrating that the performance of the composite was better than that of pristine UiO-66 and the commercial anion exchange resin Amberlyst A26.71 Inspired by the improved performance from the “ship in a bottle” strategy for producing PIL–MOF composites, other nanoporous materials, e.g., COFs and porous membranes, were also utilized as hosts for PIL.72 Kang and coworkers in situ polymerized a 1,4-bis[3-(2-acryloyloxyethyl)imidazolium-1-yl]butane TFSI monomer in a 1-ethyl-3-methylimidazolium TFSI (EMITFSI)-based electrolyte within a PDADMA–TFSI porous membrane. The well-designed hierarchical composites were used as solid electrolytes and they exhibited superior cycling performance with high specific capacities.73 Despite limited examples so far, considering the large variety of meso/microporous materials, there are great opportunities to create new PIL composites with complex structures and advanced properties. In particular, polymerization in nanoconfinement can synthesize structure-controlled PILs, which in combination with a porous host may generate myriad properties as a result of structural synergies.
Polymerization of an IL monomer bound to a substance is an alternative for developing PIL composites. In contrast to the above-mentioned porous host, which can only accommodate guests of certain sizes, i.e., the size of the IL monomer must be smaller than the pore size of the host, the current method increases the number of usable substances. Zhang et al. developed a series of cyclopropenium–ion hybrid PIL composites by RAFT polymerization.74 The materials exhibited high ion conductivity and thermal stability owing to the nature of their cationic moieties. Additionally, an IL monomer bearing a catalytically active metallosalen unit can be incorporated by free radical copolymerization into the final PIL–metallosalen composite for heterogeneous catalysts.75 It is worth noting that the surface-initiated ATRP technique is particularly effective for building PIL chains with a precise length to functionalize a variety of substrates.76–80 For instance, PIL brush-immobilized mesoporous silica by surface initiated-ATRP exhibited enhanced CO2 adsorption capacity due to the synergy of chemical and physical adsorption by PIL and silica, respectively.80 By varying the chain length of the grafted polymer on nanosilica, tunable ion conductivity for the hybrid NPs could be achieved.79 In addition, the PIL brush-functionalized electrode surface, silicon wafer or nanofiber by the ATRP technique can tune surface wettability by exchanging the counteranions of the brushes for those that are more hydrophilic or hydrophobic, which is useful for sensing and electrochemical switching applications.76–78
GO with abundant oxygen-containing functional groups is easily reduced into rGO in an imidazolium PIL solution, which is further dispersed by the PILs and used as an adsorbent for the selective isolation of an acidic protein.81,82 In particular, the use of conjugated polyfluorene PILs, which are composed of one electron-delocalized planar backbone and two hexyl flexible side chains ending with 1-methylimidazolium bromide moieties, can enhance π–π interactions with rGO sheets; this effect is due to their conductive backbones compared with those of nonconjugated PILs. The PIL sitting on the graphene sheets can prevent their agglomeration during reduction, and the imidazolium bromide ion pair can improve the electrode surface wettability for better electrochemical performance.83
In addition to the in situ chemical reduction to prepare PIL composites, the in situ growth of nano/microsized crystals such as MOFs in PILs based on a coordination-driven scenario is efficient to prepare PIL composites. Li and coworkers prepared a uniform ZIF-8/poly(4-styrene sulfonate) membrane via a layer-by-layer method and the use of a coordination-driven self-assembly strategy.84 Hydrolyzed polyacrylonitrile (PAN)-bound Zn2+ was dipped into a Zn2+ solution, and a mixed solution of 2-methylimidazole (Hmim) and poly(4-styrene sulfonate sodium salt) was alternatively added to generate a multilayer hybrid membrane. This process significantly suppressed the agglomeration of MOF particles in comparison to the co-blending method, especially at high loadings. Consequently, it exhibited an outstanding capability for removing dye from water, with high retention and good flux observed toward methylene blue far beyond those of previously reported hybrid membranes. Although the in situ coordination-driven approach is effective for incorporating nanocrystalline MOFs into PILs, control over the crystal size and MOF composition throughout the composites remains a challenge. Our group developed a coordination replacement protocol relying on a morphological replication procedure that employed a prefabricated ionically crosslinked porous gradient PIL membrane as a reactive template and precursor in a metal ion-containing solution. The kinetics and spatial coupling between the breaking of imidazolium-carboxylate ionic bonds and the immediate formation of Cu-carboxylate coordination bonds produced PIL–MOF (HKUST-1, MOF-74, and Cu-BDC) hybrid membranes with well-grown, dispersed crystals.57 Moreover, the gradient distribution of the size and mass of the MOF crystals throughout the membrane cross-section from top to bottom was well controlled, which was hard to achieve by the direct co-blending of PIL and MOF particles.
4. PIL composites and their applications
4.1 PIL–metal nanoparticle composites
MNPs are a subject of global research interest due to their diverse applications in various fields. The stabilization of MNPs is of great concern to avoid their agglomeration during their applications. Employing PILs as stabilizers has gained increasing attention over the past decade due to the following merits: (1) imidazolium/triazolium-type PILs can stabilize most MNPs by associating conventional coordination binding sites in the heterocyclic cations with the in situ generated polycarbene structure; (2) PILs as ionic polymers can strongly and selectively interact with oppositely charged species in specific applications; (3) PILs have adaptive solubility that can expand the operation window; and (4) PILs are for the most part chemically and thermally stable and easy to recycle.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and a turnover frequency (TOF) of 250 h−1.85 Groppo and coworkers compared the catalytic performance of nonionic imidazole and ionic imidazolium polymer–Pd NP composites. The PIL–immobilized Pd NPs displayed a better chemoselectivity due to the ionic environment provided by the PIL scaffolds.86 The influence of PIL substituents on catalysis was studied by Lovelock and coworkers. A series of heteroatom donor (PPh2, OMe, NH2, CN, and pyrrolidone)-modified styrenic PILs were used to synthesize PIL–Pd NP composites to catalyze the Suzuki–Miyaura cross-coupling reaction.87 In particular, the TOF of the optimized system reached 16
000 and a turnover frequency (TOF) of 250 h−1.85 Groppo and coworkers compared the catalytic performance of nonionic imidazole and ionic imidazolium polymer–Pd NP composites. The PIL–immobilized Pd NPs displayed a better chemoselectivity due to the ionic environment provided by the PIL scaffolds.86 The influence of PIL substituents on catalysis was studied by Lovelock and coworkers. A series of heteroatom donor (PPh2, OMe, NH2, CN, and pyrrolidone)-modified styrenic PILs were used to synthesize PIL–Pd NP composites to catalyze the Suzuki–Miyaura cross-coupling reaction.87 In particular, the TOF of the optimized system reached 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 h−1, which was one of the highest reported for Pd NP-catalyzed Suzuki coupling reactions between 4-bromoacetophenone and phenylboronic acid in aqueous media. The ultrasmall size and stability of NPs that was induced by the multicoordination between the PIL and the metal cores, as well as better accessibility to the active metal sites, were responsible for such a high catalytic performance.
300 h−1, which was one of the highest reported for Pd NP-catalyzed Suzuki coupling reactions between 4-bromoacetophenone and phenylboronic acid in aqueous media. The ultrasmall size and stability of NPs that was induced by the multicoordination between the PIL and the metal cores, as well as better accessibility to the active metal sites, were responsible for such a high catalytic performance.
Tailor-made nanoreactors with a judicious choice of composition have shown superior performance in accelerating kinetics, enhancing selectivity, and improving the recyclability of catalysts in many traditional organic solvents. A PIL in a micelle-like amphiphilic vesicular shape can form a nanoreactor after being incorporated with MNPs. Recently, we developed a polytriazolium (denoted as P(triaz)) PIL bearing a hexyl substituent, which spontaneously assembled into nanovesicles upon dissolution; additionally, it was capable of exerting strict control on the size of metal clusters (MCs), keeping them at ∼1 nm (Fig. 1). The association of the traditional binding power of PILs toward metal species with a finely tuned hydrophilicity/hydrophobicity balance and an in situ formed poly(N-heterocyclic carbene) (polyNHC) amplified their capping power. An as-synthesized Rh/PIL catalyst exhibited a high activity in a methanolysis reaction of ammonia borane.55 Furthermore, a hybrid nanoreactor composed of a hydrophobic organic core and a hydrophilic porous silica shell was fabricated from a PIL and mesoporous silica. The Pd NPs encapsulated in such nanoreactors showed significantly enhanced activity and selectivity in the oxidation of primary aromatic alcohols to their corresponding aldehydes in water at a mild temperature (65 °C) and pressure (2 atm). The tailor-made structural design can overcome the common accessibility problem between the substrate and the catalytically active sites; this is due to the unusual solution properties of PILs, which feature a targeted, site-specific enhancement of a hydrophobic substrate in the designed nanoreactor.88 Alternatively, Dai and coworkers developed crosslinked porous PILs (SBET of 107–132 m2 g−1) that immobilized Au NPs with an average size of 1–2 nm as a nanoreactor; excellent catalytic activity and selectivity toward aerobic oxidation of saturated alcohol were achieved with a TOF of up to 2064 h−1.89
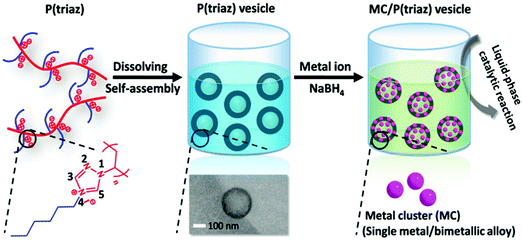 | ||
Fig. 1 Synthetic schematic of metal clusters stabilized by structurally well-defined 1,2,4-triazolium P(triaz) vesicles in a dichloromethane and methanol mixture (volume ratio = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Reprinted with permission from ref. 55. Copyright 2017 American Chemical Society. 1). Reprinted with permission from ref. 55. Copyright 2017 American Chemical Society. | ||
To improve the catalytic performance of these hybrid materials, researchers have improved and optimized the chemical composition and molecular arrangements. Compared with bulk reactions, chemical processes performed in microfluidic reactors possess the merits of low consumption of reagents, precise selection of products, and improved energy efficiency; thus, the microfluidic reactors show promise when extending the utilization of supported catalysts from conventional flasks to continuous-flow synthesis. Recently, Zhang and coworkers developed a microfluidic reactor functionalized with Au NP-porous imidazolium PIL composites that exhibited a high conversion activity and selectivity in the reduction of nitrobenzene derivatives; the high catalytic performance stems from improved contact between the substrate and the active sites of Au NPs by surface confinement.90
In addition to a one-pot catalytic reaction, the PIL–MNP nanoreactor is also useful in complicated catalytic reactions, such as cascade reactions that benefit from the synergistic effects between the active sites of PILs and metals. For example, a hierarchical organization of PIL–Au NP reactors has been developed as a bifunctional catalyst in which the IL-like units promoted Knoevenagel condensation through a dual electrophilic/nucleophilic activation relay mechanism and the MNPs catalyzed the reduction of the functional groups of the Knoevenagel product in a one-pot tandem reaction (Fig. 2).91 The structural analysis revealed that appropriately controlling the self-assembly and self-organization of the constitutive building blocks during their synthesis can optimize the microenvironments for each reaction, determining the diffusion of the intermediates from the shell to the core and out from the core to the reaction media.
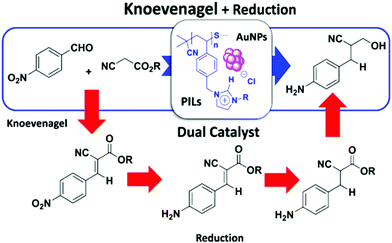 | ||
| Fig. 2 Dual catalysis based on PIL–Au NP composites for the Knoevenagel/hydrogenation one-pot tandem reaction. Reprinted with permission from ref. 91. Copyright 2016 American Chemical Society. | ||
PILs as effective stabilizers can import “smart” functions such as stimulus-responsive activity, selectivity, and substrate enrichment effects. We developed a fluidic PIL with a low Tg of −57 °C by free radical polymerization of an acrylate-type IL monomer. The synthesized PIL was amphiphilic and acted as a solvent and reaction medium in which highly dispersible and stable Pt NPs with an average size of 3.5 ± 0.4 nm could be obtained in the presence of ethylene glycol as a reducing agent.92 The as-synthesized Pt NPs were exemplified by hydrogenation of the nitroaromatics reaction, in which both hydrophilic and hydrophobic substrates performed well. Such work pointed out the potential application of PIL composites as environmentally friendly, solvent-free and homogeneous fluidic catalysts. Sreedhar and coworkers demonstrated a reversible “solubility switch” of PIL–Ag NPs by an anion metathesis reaction through which the dispersibility of NPs could be easily changed from an aqueous to an organic medium and vice versa.93 The change in the dispersibility of MNPs facilitated MNP-mediated homogeneous and heterogeneous catalysis. Alternatively, Harandi and coworkers prepared a pyridinium PIL copolymer bearing a thermoresponsive block poly(N-isopropylacrylamide) (PNIPAM) (denoted as PNIPAM-b-PIL).94 Once incorporated with Pd NPs, such a PIL–Pd composite showed an inherent thermoresponsiveness in C–C coupling reactions that were triggered by the LCST phase transition; above the LCST, the collapsed PNIPAM-b-PILs offered a high concentration of substrates around the Pd NPs.
In addition to stimuli-responsive catalysis, PIL–MNP composites can be used as media for stimuli-responsive optical/electronic materials. An example was illustrated in the PIL–Au composite (PIL = poly(1-decyl-3-vinylimidazolium chloride)), in which a lamellar polymer mesostructure was detected in a dried state (Fig. 3). Upon swelling in alcohol, the composite underwent a structural conversion to a disordered state, accompanied by a color change from purple to pale pink and a shift in the surface plasmon resonance to 527 nm, which is consistent with isolated, noninteracting particles. Such a color evolution arose primarily from the synergy of polymer-matrix-assisted changes in the NP packing order and interparticle spacing.95
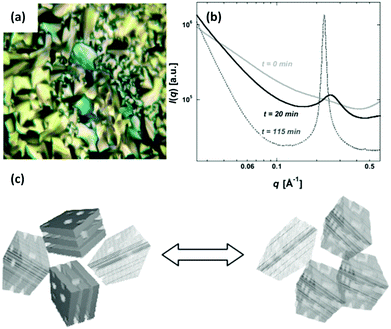 | ||
| Fig. 3 (a) Polarized optical image (10×) of liquid crystalline textures collected on an in situ formed poly[C10VIm+][Cl−] in the presence of a 15 ± 1% (w/w) aqueous HAuCl4 solution. (b) Averaged values of small-angle X-ray scattering (SAXS) data collected at selected time intervals during in situ deswelling experiments. (c) Schematic illustration of the structural variation between the mosaic distribution of randomly perforated lamellae and in-plane hexagonal ordering of channels in the lamellae of the polymer composite during solvent evaporation (deswelling). Reprinted with permission from ref. 95. Copyright 2007 Wiley-VCH. | ||
In addition to magnetic Fe3O4 NP-based PIL composites, other functional metal oxide NPs, e.g., VxOy, TiO2, and La2O2CO3 with controlled shapes and sizes, have also been utilized as constituent materials for PIL composites.99,100 Recently, Yan and coworkers reported a PIL composite doped with IL-tethered TiO2 NPs as a gel electrolyte in quasi-solid-state dye-sensitized solar cells (DSSCs). These DSSCs demonstrated improved device performance with an enhanced short circuit current density (Jsc) and open circuit voltage (Voc) compared with DSSCs with unmodified TiO2 NPs (Fig. 4). The effective Grotthuss conduction mechanism enhanced the ionic conductivity and diffusion coefficients of I3 and contributed to improved performance. These results also indicated that the cells based on PIL–TiO2 hybrid electrolytes could overcome the drawbacks of volatile liquid electrolytes and offer a feasible method to fabricate quasi-solid-state DSSCs in future practical applications.101
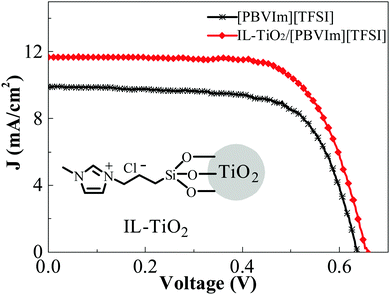 | ||
| Fig. 4 J–V curves for the DSSCs assembled with different gel electrolytes under a simulated air mass 1.5 solar spectrum irradiation at 100 mW cm−2. Reprinted with permission from ref. 101. Copyright 2012 Elsevier. | ||
The combination of PILs and MNPs can even generate a composite with functions different from the individual components. For example, poly[(p-vinylbenzyl)trimethylammonium PF6] and La2O2CO3 NPs—both of which are intrinsically insulating materials—were utilized as building blocks to fabricate a composite film by ex situ drop-casting on supports. The composite took full advantage of the electrostatic interaction at the interface, which boosted the overall conductivity of the composite at room temperature (Fig. 5). The as-prepared film, with NP loading between 60 and 80 wt%, showed an improved overall conductivity due to the high mobility of PF6− anions around the NPs, which was derived from the electrostatic interactions between the cationic PILs and the negatively charged La2O2CO3 NPs. The rearrangement of tetraalkylammonium cations in the presence of CO2 was responsible for the enhanced conductivity, which was further demonstrated by an increase in the conductivity when exposed to pulses of CO2 between 150 and 2400 ppm at room temperature and a relative humidity of 50%. The PILs here behaved as a tool to subtly engineer conductive channels localized at the interface by a synergistic interaction between the PILs and NPs.102
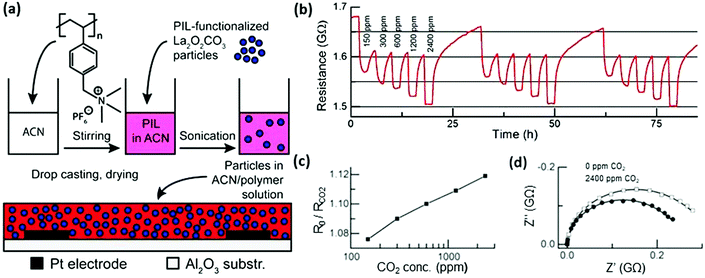 | ||
| Fig. 5 (a) Sensing performance of thin films composed of 70 wt% La2O2CO3 NPs at 50% relative humidity in air and at room temperature. (b) DC resistance change during exposure to CO2 pulses between 150 and 2400 ppm. (c) Corresponding sensor signals, calculated as resistance in air divided by resistance under CO2. (d) Nyquist plots with (circles) and without (squares) 2400 ppm CO2. Measured data (symbols), fitted curve (solid line). Reprinted with permission from ref. 102. Copyright 2015 Wiley-VCH. | ||
As a photoconductive semiconductor, bismuth sulfide (Bi2S3) has a direct band gap (Eg) of approximately 1.3 eV, which approaches the ideal value (1.34 eV) for maximum photovoltaic efficiency. The present approaches for preparing Bi2S3 overwhelmingly describe one-dimensional (1D) structures because of their highly anisotropic crystal structure that consists of infinite chains of covalently bound atoms. Gao et al. used several PILs as additives to modify the nucleation and growth of Bi2S3 materials with tailored sizes, dimensions, and architectures (nanowires, hexagonal plates, and mesoporous sheets); consequently, a tunable Eg (from 1.54 to 1.95 eV) was observed owing to the quantum-size effect (Fig. 6a).54 Intriguingly, the PIL-modified Bi2S3 nanowires can efficiently catalyze water oxidation, with an onset potential of OER occurring at approximately 1.46 V versus a reversible hydrogen electrode (RHE); however, the commercial counterpart is catalytically inert (Fig. 6b–d). It is proposed that the PIL chains grafted on Bi2S3 can modify its surface electronic structure, which leads to an oxidation state of Bi that has a high formal charge. Such merits further improve the catalytically active sites on Bi2S3 for optimum O-intermediate adsorption.
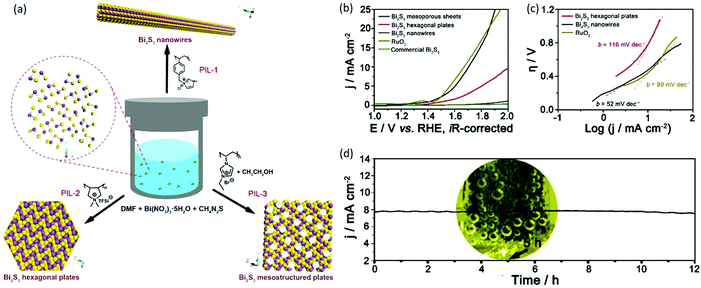 | ||
| Fig. 6 (a) Bi2S3 crystals with morphological diversity prepared by using PILs as additives. (b) Polarization curves for OER on modified FTO electrodes comprising PIL-stabilized Bi2S3 crystals. (c) Tafel plots for various catalysts derived from (b). (d) Chronoamperometric response (j–t) recorded from the as-prepared Bi2S3 nanowires at a constant overpotential of 400 mV. Inset: Photograph showing O2 bubbles accumulated on the Bi2S3-modified FTO electrode. Reprinted with permission from ref. 54. Copyright 2016 Wiley-VCH. | ||
4.2 PIL–carbon composites
Carbon materials with excellent mechanical performance offer a number of advantages, namely, variable electron conductivity, good thermal conductivity, and chemical resistivity. In the past two decades, carbon materials, such as carbon nanotubes (CNTs), graphene, and fullerene, have attracted enormous attention ranging from material breakthroughs to diverse practical applications in electronics, optics, catalysis, and biology. However, most carbon nanomaterials without protection easily aggregate in solution, which limits their processing into high-performance devices. To achieve high loading and homogeneity of carbon-based composites, defects in carbon (nano)materials are usually introduced by chemical functionality, sometimes resulting in an unfavorable deviation from their original properties. To integrate desirable properties of carbon materials without trading performance, PILs are employed as noncovalent dispersants for carbon materials in various solvents for producing devices by solution processing. In addition, the integration of PILs and carbon materials also creates new functions due to synergistic effects.PIL–CNT composites have been extensively studied for energy storage and conversion. In 2009, Chen and coworkers reported a strategy to functionalize CNTs by in situ thermally initiated radical polymerization of an IL monomer, 1-ethyl-3-vinylimidazolium tetrafluoroborate, on a CNT surface.104 CNTs covered by PILs can disperse in water and further serve as a medium to immobilize PtRu NPs (Fig. 7). Such PIL–CNT–PtRu composites electrooxidized methanol better than PtRu/CNTs without PILs as a surface modifier. This work points out that it is promising to use PILs as a “surface modifier” to incorporate with CNTs for enhanced performance. Alternatively, Heumann and coworkers used PIL-modified CNTs for scalable synthesis of single dispersed cobalt ions that were coordinated by PIL. The composites were tested as excellent catalysts for the oxygen evolution reaction (OER) because of the variations in the electronic structure of Co by coordinating with PIL, as well as the excellent conductivity contributed by CNTs.105
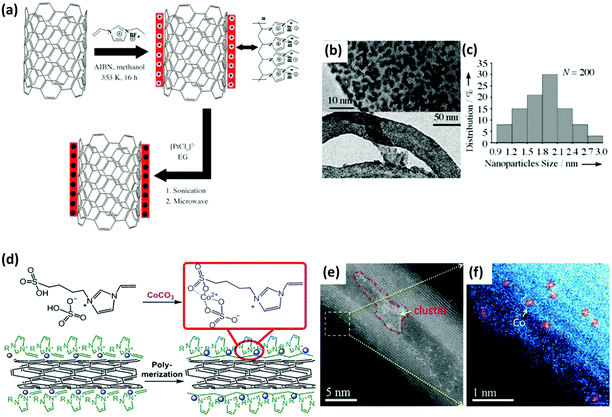 | ||
| Fig. 7 (a) Synthetic scheme toward Pt/CNT–PIL nanohybrids. EG: ethylene glycol, AIBN: 2,2′-azobisisobutyronitrile. TEM images (b) and size distribution plot (c) of Pt NP/PIL nanohybrids. (d) Preparation scheme toward CoSSPIL/CNT. The blue spheres represent single Co species. The Scheme only shows the cation part on the CNT before and after polymerization. (e and f) HAADF-STEM images of CoSSPIL/CNT-1. Reprinted with permission from ref. 104 and 105. Copyright 2009 and 2018 Wiley-VCH. | ||
The synergistic effect between PIL and CNTs was clearly illustrated in a report by using redox-active catechol-bearing PIL–CNT composites as high-performance lithium-ion battery cathodes. The composite delivered a high reversible capacity of 247 mA h g−1 at 0.2C with a capacity of 134 mA h g−1 at 60C and retained 86% of its initial capacity during the prolonged 5000 cycling tests in comparison to a neutral polymer PAmcat in control experiments (Fig. 8). In comparison to the catechol units in the neutral polyacrylamide, the catechol moieties in the polyvinylimidazolium backbone bearing the TFSI− counteranion were anticipated to impart the following enhancements in Li-storage performance: (1) strong bonding between catechol PILs and CNTs via π–π/π–cation interactions, (2) superior cyclability without leaching of catechol groups into the organic electrolyte, (3) improved discharge potential due to electron-deficient imidazolium units in PILs, and (4) enhanced rate capability caused by favorable anion mobility at the electrolyte–electrode interface and in the bulk of the electrode.106
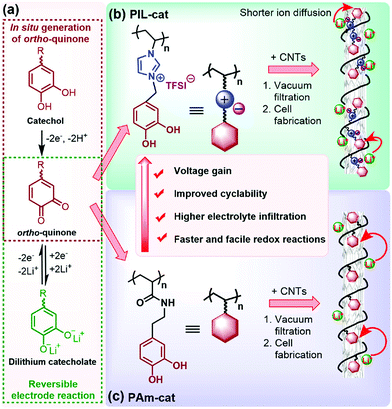 | ||
| Fig. 8 Construction of catechol-based PIL/CNT buckypapers for LIBs. (a) ortho-Quinone/dilithium catecholate redox reaction. (b and c) Enhancement in the electrochemical performance of cationic PIL-cat (b) compared to its neutral counterpart PAm-cat (c). Reprinted with permission from ref. 106. Copyright 2018 American Chemical Society. | ||
In addition to using PIL–CNTs for energy-related applications, such conducting composites can also be applied in electromechanical devices such as “smart” actuators. It has been revealed that enhanced conductivity and flexibility with high thermal stability and mechanical durability can be regulated by the weight content of CNTs in PIL–CNT composites.107 The disclosed properties favor the potential application of PIL–CNT composites in electromechanical devices. Ricci and coworkers prepared dry electrochemical actuators using an imidazolium PIL and CNT composite gel as the electrode.108 A direct correlation between the size of the anion (Br−, BF4−, and TFSI−) in the electrode and the resulting strain of the actuator could be detected, i.e., large anions lead to high strain. Consequently, the displacement response of the actuator was proportional to the size of the counteranions. A maximum bending displacement of up to 0.5 mm was recorded under a low applied voltage of ±1 V. Alternatively, Lin et al. developed a porous PIL membrane composite with aligned CNTs in its matrix and realized programmable, anisotropic actuation toward organic vapors. In this case, the actuating direction can be well predicted at a direction perpendicular to the longitudinal orientation of CNTs.109
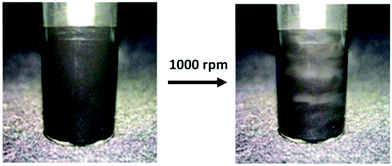 | ||
| Fig. 9 Photograph of a vial containing PIL-stabilized graphene dispersion with an aluminum cylindrical stirrer (left) at rest and (right) at 1000 rpm. This change in reflectivity illustrates an anisotropic to a nematic transition. Reprinted with permission from ref. 18. Copyright 2014 American Chemical Society. | ||
The excellent dispersibility of the PIL–graphene composite allows for deposition on a broad range of substrates, including electrodes that can harness the electric properties of graphene for various sensing purposes. For example, Li and coworkers prepared a PIL–rGO composite as an electrochemical sensor for phenylethanolamine (PEA) determination at a high sensitivity. Comparing a PIL sensor and a bare electrode, the PIL–rGO sensor had the highest peak current response for PEA with a low detection limit of 0.002 μM due to the synergistic effect between rGO and PIL.110 Similarly, such a PIL–GO composite system can also sense dopamine.111
PIL–graphene composites were explored as high-performance supercapacitors and were pioneered by Ruoff, Suh and coworkers.51 These composites displayed a stable electrochemical response up to 3.5 V operating with a maximum energy density of 6.5 W h kg−1 and a power density of 2.4 kW kg−1. PILs coated on GO enhanced the compatibility of the composite with the IL electrolyte, i.e., better wettability of the IL electrolyte on the composite materials, which synergistically increased the effective electrode surface area that was accessible to the electrolyte ions.
When graphene functions as a platform to offer a high specific surface area in the PIL–GO composite, the PILs can selectively collect the countercharged species, enabling selective isolation/separation of substrates. Nassernejad et al. developed a promising candidate for DNA delivery based on a PIL–3D GO framework. This PIL–GO composite has a strong electrostatic binding capability to a nucleic acid and a negatively charged cell membrane. Synergistically, the positively charged PIL–GO composite loaded with DNA of a small size was favorable for cellular uptake and intracellular trafficking, facilitating the use of the 3D composite as a suitable carrier for DNA delivery.112 Alternatively, covering the SiO2 surface with PIL–rGO composites can allow selective adsorption of acidic proteins such as ovalbumin due to the high specific surface area and the available electrostatic and π–π interactions.82
GO-Based mixed matrix membranes (MMMs) capable of anion exchange are scarcely explored in the literature. Szekely, Budd and coworkers prepared a series of PIL–GO nanocomposite anion exchange membranes (AEMs) with low GO loadings. The AEMs exhibited a high ion exchange capacity of 1.7–2.1 mmol g−1, a good permselectivity of up to 0.99, and a relatively low area resistance that goes down to 2.9 U cm−2. A trade-off between good selectivity and low resistance was investigated for membranes with low GO loadings (0.25–2.5%). The GO-based nanocomposite AEMs demonstrated excellent potential for electrodialysis with a permselectivity of 0.99 at a loading content of 1% GO.113
4.3 PIL–silica composites
Silica is cheap and chemically inert, and allows for irreversible surface modification through surface silanol groups. The functionalization of silica with a polymer can exhibit dramatic improvements in strength, modulus, heat resistance, gas permeability barrier properties and so on. One of the advantages of using PILs with diverse structures and functions to coat silica is to modify its surface characteristics, especially its hydrophilic/hydrophobic properties. It is well known that surface properties are important for the interactions between the material and the environment. In particular, wettability control has attracted extensive interest in the development of “smart” devices, e.g., self-cleaning devices, discrete liquid droplet manipulators or tunable optical lenses. By immobilizing a poly[1-(2-acryloyloxyhexyl)-3-methylimidazolium bromide] brush layer onto the electrospun SiO2 nanofiber surface via an SI-ATRP technique, an anion-directed molecular gating system was achieved. The use of a counteranion exchange reaction allowed the surface properties of the membrane to be reversibly altered between hydrophilic and hydrophobic; it enabled pores to withdraw or expel solvent molecules (H2O), thus controlling the transport of probe molecules through the membrane (Fig. 10).78 Similarly, the wettability of glass/silicon surfaces can be modulated by grafting different PILs.114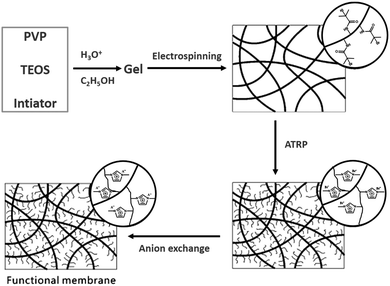 | ||
| Fig. 10 Schematic illustration of the preparation of the PIL brush-coated electrospun silica–PIL composite membrane. Reprinted with permission from ref. 78. Copyright 2010 Royal Society of Chemistry. | ||
In addition to the surface modification of silica by PIL, silica serving as a nonconducting filler can increase the conductivity of both semicrystalline and amorphous polymer electrolytes, which provides the possibility of generating complementary functionality (i.e., structural stability) and transport tunability for ion-conducting materials. For example, Ye and coworkers reported a PIL-functionalized mesoporous silica nanoplate, which increased the ionic conductivity of the PIL/IL electrolyte by 1130% at ∼30 °C because of a significant decrease in the ion transport activation barrier (approximately 10 kJ mol−1). Such nanofillers simultaneously conferred both a high ionic conductivity of ∼10−3 S cm−1 at 130 °C with only 15 wt% IL loading and excellent IL immersion and retention properties to the mixed PIL/IL polymer electrolytes. The nature of the disposition and the ionic channel junctions, formed through nanoscopic modification, is expected to facilitate ion transfer through the ionic channels.115 It can also be extended to other PIL–silica composites such as pyrrolidinium-based PIL–silica hybrid ionogel electrolytes (HIGEs)116 or core–shell SiO2–poly[p-vinylbenzyl-trimethylammonium tetrafluoroborate] (SiO2–P[VBTMA][BF4]) composites with tunable structures as high-performance ion-conducting systems.117
The combination of nanoporous silica with PIL can serve as a functional porous material for various adsorption and separation events, including CO2 capture, antidiabetic drug extraction, ion exchange, and reversed-phase and liquid chromatography. The presence of ions on PILs enhances CO2 absorption capabilities, which can be further improved by a nanoporous silica component in the composite. For example, P[VBTMA][BF4]-functionalized mesoporous silica (meso-silica) hybrids can rapidly adsorb CO2 at a capacity of 0.4 mmol g−1 at 30 °C from a simulated flue gas containing 10 vol% CO2.118 Similarly, polymerizing 1-(2-acryloyloxyundecyl)-3-methylimidazolium bromide ([mC11C1Im]Br) that is already attached onto a mercaptopropyl-functionalized silica Sil-MPS results in a hybrid Sil-pC11C1Im. The carbonyl, polar imidazolium, and hydrophobic alkyl chain groups in the composite as interaction sites show a considerably high molecular-planarity selectivity toward polycyclic aromatic hydrocarbon isomers, additional anion-exchange capability and available hydrophilic interaction.119 Moreover, silica-coated iron oxide NPs modified with an imidazolium PIL (Fe3O4@SiO2@PIL) were used as a sorbent for magnetic solid-phase extraction (MSPE) and determination of trace amounts of antidiabetic drugs in human plasma. The modification of PILs combined the magnetic response of NPs with IL characteristics and improved the stability, reusability, mass transfer capacity and diffusion routes of the nanoscale sorbent.120
4.4 PIL–metal salt composites
Metal salts can easily complex with PILs via electrostatic/coordination interactions. To date, the most extensively investigated PIL–metal salt composites are the main group of metal–PIL systems, such as Li+/Na+–PIL. When applied in Li-ion batteries as solid electrolytes, PILs are usually combined with IL-based electrolytes (a solution of Li/Na salts in ILs) to enhance their inherently limited ion conductivity. The chemical affinity between PILs and ILs prevents them from phase separation; additionally, in comparison with traditional solid electrolytes, the resulting composite electrolytes are provided with outstanding integrated performances, including a high ion conductivity, a wide electrochemical window, a favorable plating morphology of the Li metal, and a device that does not leak and is noncombustible at room temperature.68 Zhou et al. fabricated a hierarchical PIL-based solid electrolyte (HPILSE) by integrating a PDADMADTFSI porous membrane and a polymerized 1,4-bis[3-(2-acryloyloxyethyl)imidazolium-1-yl]butane TFSI (C1-4TFSI) cross-linking network within an IL 1-ethyl-3-methylimidazolium TFSI (EMITFSI)-based electrolyte, where the cross-linked C1-4TFSI maintained the entire mixture in a quasi-solid-state to avoid liquid leakage even at high temperature. The as-prepared PDADMADTFSI porous membrane and a suitable amount of the EMITFSI-based electrolyte provided a tensile strength of 2.4 MPa and an ion conductivity above 10−3 S cm−1 at 25 °C. Because of the robust interfacial contact between the HPILSE and electrodes going against the electrode volumetric changes during charge/discharge cycles, when this system is applied in Li-ion and Na-ion batteries, better electrochemical performances can be achieved for the assembled LiFePO4/HPILSE/Li and Na0.9[Cu0.22Fe0.30Mn0.48]O2/HPILSE/Na polymer cells (Fig. 11).73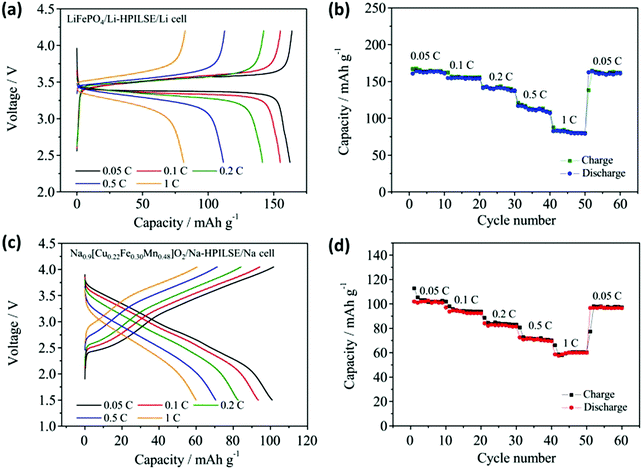 | ||
| Fig. 11 Electrochemical characterization of Li-ion batteries exploiting HPILSE. (a) Typical charge and discharge curves and (b) rate performance of the LiFePO4/Li-HPILSE/Li cell. Electrochemical characterization of Na-ion batteries exploiting HPILSE. (c) Typical charge and discharge curves and (d) rate performance of the Na0.9[Cu0.22Fe0.30Mn0.48]O2/NaHPILSE/Na cell. Reprinted with permission from ref. 73. Copyright 2017 Elsevier. | ||
In addition to being employed as solid-state electrolytes, PIL-immobilized metal salts containing both metal centers (Lewis acids) and ionic units (nucleophiles) can serve as bifunctional catalysts in heterogeneous reactions. The combination of nanobelt α-CuV2O6 with a porous PIL derived from a crosslinked solid acid P(EVPI-Br) (Fig. 12) generated a binary catalyst and afforded a 63.1% yield in the one-pot one-step conversion of fructose into 2,5-diformylfuran (O2, 135 °C, 3.5 h). The hydrophilic surface of P(EVPI-Br) enabled the preferential adsorption of fructose on the acid sites; in contrast, α-CuV2O6 strongly adsorbed 5-hydroxymethyl furfural (HMF) but had weak affinity to fructose (Fig. 12). Such surface wettability-controlled adsorption features inhibited the oxidation of fructose and facilitated the transfer of HMF to the oxidative sites on α-CuV2O6, and thus, its timely conversion into the final product.121 Similarly, the PIL–metal salt hybrid provides an ideal platform to produce diverse binary catalysts for task-specific catalysis.75 For example, introducing CrCl3·6H2O into SO3H-functionalized poly(1-vinyl-3-propanesulfonylimidazolium) (PVMPS) with PW12O40 and Cl as counteranions provides bifunctional catalysts carrying Brønsted/Lewis acidic groups, which effectively convert biomass including fructose, glucose, and cellulose into HMF in the presence of DMSO-mediated solvents.61
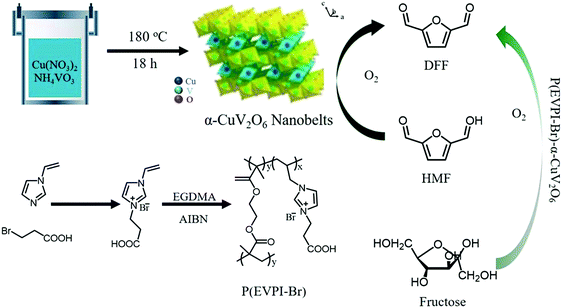 | ||
| Fig. 12 Synthesis of α-CuV2O6 and MPIL P(EVPI-Br) for the conversion of 5-hydroxymethyl furfural (HMF) and fructose into DFF. Reprinted with permission from ref. 121. Copyright 2017 Royal Society of Chemistry. | ||
The incorporation of metal ions into PIL-containing membranes can also influence the gas separation process. The metals sometimes can serve as carriers for enhanced affinity to certain gases; for example, a high ethylene absorption capacity can be achieved by a mixture of PILs containing the [TFSI] anion and the AgTFSI salt.70 Bara and coworkers recently explored PIL–IL membranes containing the [CuCl2]− anion as a gas separator, in which enhancement of both gas permeability and ideal gas pair selectivity was observed for CO2/N2 and H2/N2 separation in the composite membranes with respect to the neat poly([C4VIm][TFSI]). The [CuCl2]− anion was able to selectively and reversibly bind CO2 molecules to increase the solubility of CO2 in the composite.122
In addition to the aforementioned applications, PILs can synergistically work with functional metal sites to develop “smart” hybrids or devices.123–129 By incorporating a redox-active metal site into a polyferrocenylsilane derivative (PFS–SO3), Vancso and coworkers developed organometallic PFS–PIL hybrids for electrochemical sensing (Fig. 13), switchable porous materials, plasmonic NPs and smart windows due to their redox- or electrical-responsive behaviors.126 Moreover, by introducing β-CD into this PIL–ferrocene composite system, a “smart” velcro-mediated polymer could be achieved by responses to redox stimuli. The synergistic effect between the PIL and PFS created a family of “smart” composites for broad areas.128,129
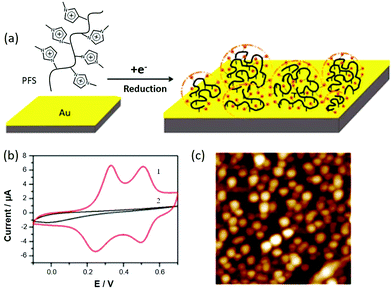 | ||
| Fig. 13 (a) Schematic illustration of the electrografting of PFS-MID-Cl in ILs. (b) Cyclic voltammogram of a PFS-modified Au electrode (1) with a deposition potential of −2.2 V and (2) with a deposition potential of −0.8 V in 0.1 M NaClO4; this was conducted using an Ag/AgCl reference electrode and a Pt counter electrode at a scan rate of 50 mV s−1. (c) AFM height image of PFS grafts on Au using a tapping mode in air; scan size: 1 μm × 1 μm, z-scale: 30 nm. Reprinted with permission from ref. 126. Copyright 2014 American Chemical Society. | ||
4.5 PIL–polyoxometalate composites
POMs, anionic metal oxide clusters with several to tens of negative charges, have been extensively investigated in catalytic applications due to their unique electrical and optical properties. However, their intrinsic hydrophilicity causes difficulties in their separation and recycling during catalysis. A number of strategies have been developed for the heterogenization of POM catalysts, including phase transformation, microemulsion formation, encapsulation by inorganic or organic cations, and immobilization into a polymer or a silica matrix. Assembly of POMs with polycations is revealed to be a successful example for preparing heterogeneous POM catalysts, which is beneficial for adjusting the solubility, redox properties, and surface microenvironment of POMs. To date, PILs have been used to pair with heteropolyanions to prepare diversified PIL–POM hybrid catalysts in various reactions, including oxidation of sulfides to sulfoxides, hydroxylation of benzene, photodegradation, epoxidation and oxidation of 5-hydroxymethylfurfural. Doherty et al. immobilized [PO4{WO(O2)2}4]3− on a pyrrolidinium-functionalized PIL to produce a [PO4{WO(O)2}4]@PIL composite, which exhibited high efficiency and long-term activity for the oxidation of allylic alcohols and alkenes compared with PIL-free [PO4{WO(O)2}4]@silica composites. These comparisons underpin the potential benefits of preparing a PIL support for robust POM catalysts in reactions.130Wang and coworkers systematically investigated PIL–POM composites in heterogeneous catalysis. They developed hierarchically nanoporous PILs as supports for immobilizing POMs. In catalytic reactions, the mesoporosity with a relatively high specific surface area benefits the loading and good dispersion of the active POM species as well as the accessibility of substrates to active sites, while the availability of both meso- and macropores favors the improvement of mass transport. For example, a hierarchical meso-macroporous PIL (HMP) was synthesized by radical polymerization of a 1-allyl-3-vinylimidazolium chloride [AVIm]Cl monomer using a tri-block copolymer P123 poly(ethylene oxide)-block-poly(propylene oxide)-block-poly-(ethylene oxide) (PEO–PPO–PEO) as a soft template; this process was followed by the removal of P123, and thus, a highly dispersed Keggin-structured phosphotungstic anion PW12O403− (PW) was immobilized inside the mesopores through anion exchange (Fig. 14a). This composite acted as a heterogeneous catalyst in the liquid-phase epoxidation of cis-cyclooctene with H2O2, showing superior catalytic activity compared with that of the phosphotungstic acid H3PW and hybrid catalyst [AVIm]–PW (Fig. 14b).131
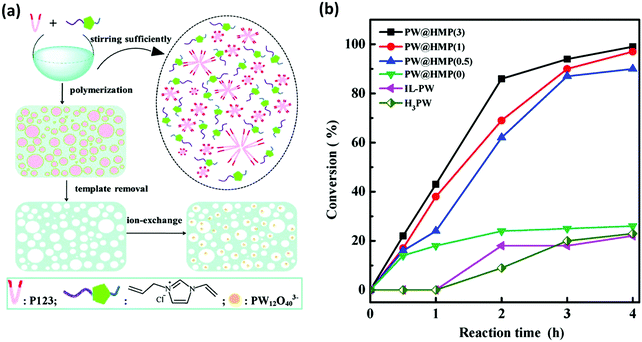 | ||
| Fig. 14 (a) Synthetic process of hierarchical meso-macroporous PILs by using the soft template P123. (b) Conversion of cis-cyclooctene as a function of reaction time for PW@HMP(x)-catalyzed liquid-phase epoxidation with H2O2. Reaction conditions: cis-cyclooctene (5 mmol), H2O2 (4 mmol, 30 wt%), methanol (1 mL, as the solvent), 60 °C, 4 h. Reprinted with permission from ref. 131. Copyright 2015 Royal Society of Chemistry. | ||
Similar hierarchically nanoporous PIL–POM systems have been explored, e.g., poly(divinylbenzene-3-n-butyl-1-vinylimidazolium) (P-[DVB-VBIM]+)-containing a Keggin HPA-anion (PMo10V2O40) or mesoporous ionic network-POM composites. These catalysts are highly active toward hydroxylation of benzene with H2O2132 and ambient selective aerobic oxidation of HMF into 2,5-diformylfuran with O2, outperforming the PIL-free POM catalysts.133
In addition to using nanoporous PILs as an advanced support for immobilizing POM catalysts, the surface modifications of POM catalytic sites are another aspect to enhance catalytic performance. In particular, the development of amphiphilic POM composites is important in heterogeneous catalysis, especially in aqueous/oil biphasic systems, to balance activity and selectivity. By harnessing the unique properties of PILs with tunable cations and/or anions, it is feasible to improve the adaptability of POMs toward the external environment for task-specific applications. Leng et al. designed a PIL copolymer containing units of dodecyl imidazolium IL (DIM) and carboxylic acid-functionalized imidazolium IL (CIM) to fabricate an amphiphilic POM–PIL composite through ion exchange with the [PO4(WO3)4]3− species. The hydrophobic alkyl chains (–C12H25) and hydrophilic carboxyl acid groups (–COOH) were introduced to supply a unique interfacial amphiphilic microenvironment for H2O2-based epoxidation of alkenes. In this case, the peroxo-tungstate species in POM anions acted as the active centers for epoxidation reactions, whereas the amphiphilic catalyst structure served as a “trapping agent” for both the hydrophobic alkene substrates and the hydrophilic H2O2 molecules. Therefore, the mass transfer in the liquid–liquid–solid media was accelerated, and the catalytic activity was promoted.134 The combination of porous PIL supports featuring an amphiphilic surface is effective for immobilizing POM catalysts. Recently, a polyhedral oligomeric silsesquioxane (POSS)-derived mesoporous composite (by copolymerization of dicationic imidazolium IL [1,1-(butane-1,4-diyl)-bis(3-vinylimidazolium)]Br2 (BM) with octa(N-vinylimidazole silsesquioxane) POSS) has been employed to combine with a Keggin-type POM, yielding amphiphilic mesostructured POM-based ionic hybrid catalysts. The mesoporous structure and the amphiphilic catalyst surface, which allow for rapid diffusion of the reactants into the reactive POM centers, are responsible for the excellent catalytic performances in the epoxidation reaction with H2O2.135
4.6 PIL–organic polymer composites
Organic polymers possess some unique attributes, including ease of production, light weight, and tunable thermal and chemical stability, which are desirable to integrate them with other functional materials for composites. PILs as functional polyelectrolytes can be combined with polymers for enhanced mechanical strength while simultaneously maintaining ionic conductivity.136 Moreover, due to the high compatibility between PILs and a broad range of polymer substances, PILs can be used to polymerize on polymer particle seeds to grow PIL–polymer composite particles. These composite particles have the advantage of low cost and easy processability in comparison to PIL homopolymer particles. In the case of the core–shell composite particle consisting of a core from a commercial polymer and a PIL shell, the films and pellets prepared from the composite particles are also promising for the design of ionic conductive materials, in which the ionic conductive PIL shell forms effective ion transport paths. Recently, such composite particles have been achieved in PIL–PMMA (poly(methyl methacrylate)) composite particles with either a sea-island structure consisting of PIL domains or core–shell PIL–PMMA structures (Fig. 15a). These results are consistent with the theoretical considerations that are based on the spreading coefficients calculated from the interfacial tensions. Moreover, hydrophobic PILs of high polarity are effective for controlling the morphologies of the final composite particles.137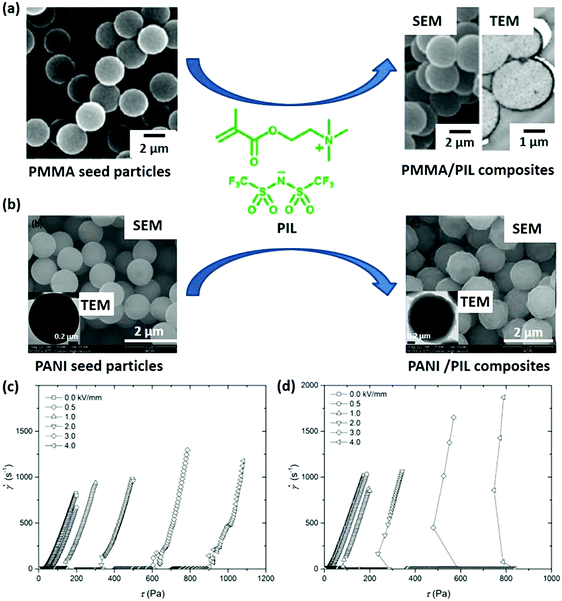 | ||
| Fig. 15 (a) PMMA (left) and PMMA/PIL (right) composite particles. (b) PANI (left) and PANI/PIL (right) composite particles. (c) Flow curves of shear rate versus shear stress for the electrorheological suspensions of PIL microspheres. (d) PANI/PIL microspheres. Reprinted with permission from ref. 137 and 138. Copyright 2013 American Chemical Society and Copyright 2018 Wiley-VCH. | ||
In addition to the morphology control of PIL–polymer composites, a synergistic effect can be engineered by placing a polymer, bearing responsive properties, within PIL composite particles for enhanced properties. For instance, a monodispersed PIL microsphere coated with a semiconducting polyaniline (PANI) shell shows an enhanced electroresponsive electrorheological effect with a lower power consumption in comparison to neat PIL microspheres (Fig. 15b).138 The authors claimed that the semiconducting PANI shell could effectively limit the irreversible ion leakage of PIL microspheres and improve the particle polarizability, resulting in decreased power consumption and an enhanced electroresponsive electrorheological effect. Alternatively, when using inherently conducting PANI as a core, they developed a type of PIL-encapsulated PANI composite particle that overcame the surface charge character of pure PIL particles and enhanced the electrorheological effect.
PILs can also directly complex with another polymer to form porous NPs. We recently reported ionic complexation between the poly(3-cyanomethyl-1-vinylimidazolium) series (PCMVImX, X = TFSI−, PF6−, BF4−, or Br− anions) and PAA that yielded insoluble aggregated nanoparticles.139 The powders of these particles possessed micro- and mesopores with an SBET and a pore volume of up to 310 m2 g−1 and 0.98 cm3 g−1, respectively. They were stable when refluxed in ethanol as well as several other organic solvents (80 °C) for 24 h. This composite was further loaded with a copper salt and served as a heterogeneous catalyst for the aerobic selective oxidation of C–H bonds.
In addition to the synthesis of composite NPs, the most obvious advantage of combining PILs with other polymers is membrane fabrication. In this process, since the PIL is an ionic polymer, the compatibility, especially the charge compensation, between the PIL and the other polymers should be carefully considered to produce a uniform composite structure that is beneficial for material performance. For example, the above-mentioned PIL–PAA complex can be extended to fabricate hierarchically structured nanoporous ionic complexation membranes. The pore character can be tuned by controlling the charge density, the molecular weight of the acids, and the additives. Such membranes have either a homogeneous porous cross-section or a gradient distribution of pore sizes.64 In addition to ionic complexation driven by electrostatic interactions, other supramolecular interactions can also be considered as a tool for composite film fabrication. An example is illustrated here by the combination of poly(vinylidene fluoride) (PVDF) with a poly(2-(dimethylamino)ethyl methacrylate) methyl chloride; in this case a film is fabricated through noncovalent interactions between the localized cationic ions of the PIL and the polar –CF2 group of PVDF, which results in a PIL–PVDF composite film featuring excellent ferroelectric properties.140
PIL–polymer membranes are useful in electrochemical devices. In particular, PIL-based electrolytes are selected for memory devices because of their advantages of mechanical stability, safety, good processability and moderate ionic conductivity. Li et al. constructed a three-terminal nonvolatile memory device by combining a poly[1-(4-vinylbenzyl)-3-butylimidazolium tetrafluoroborate] (PVBIT) PIL layer, a hydrophilic polythiophene and a poly(3-carboxypentylthiophene) (P3CPT) active layer. Memory effects could be obtained at high write/erase voltages (+3/3 V) and a low read voltage (0.5 V) in the three-terminal device. Simultaneous monitoring of both the ‘‘write’’ and ‘‘read’’ operations revealed an instantaneous response and a fast switching speed (Fig. 16). The memory behavior of the composite could be attributed to the synergistic effect between the polarization of the polythiophene backbone due to the P3CPT polaron and then stabilization through both the carboxylate anions and the BF4 anions that immigrate from the polyelectrolyte layer.141
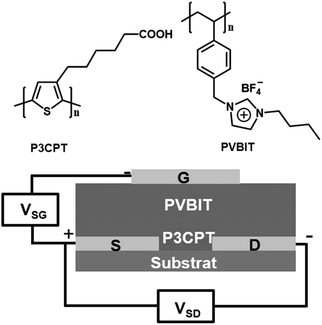 | ||
| Fig. 16 Chemical structures of the materials used in a nonvolatile memory device. Reprinted with permission from ref. 141. Copyright 2015 Wiley-VCH. | ||
The use of ILs in polymeric membranes is known to elevate the electrochemical performance of proton exchange membrane-based fuel cells (PEMFCs). However, they suffer from drawbacks such as IL drain and a decrease in mechanical properties. The incorporation of PILs is anticipated to provide a continuous pathway for the IL character (which is present in the repeating unit of the polymer), while eliminating the issue of IL drain. PILs also retain the unique properties of ILs to a certain extent (e.g., ionic conductivity, thermal stability, tunable solution properties and chemical stability), which in combination with their intrinsic polymer properties are expected to offer better benefits than simple ILs. Kharul and coworkers reported a membrane composed of homogeneously mixed PDADMA–trifluoromethanesulfonate (P[DADMA][TFMS]) and polybenzimidazole (PBI-I) for PEMFCs with a proton conductivity of up to 0.07 S cm−1 at 150 °C. Single-cell evaluations of the membrane have been successfully demonstrated using H2/O2 at 160 °C under nonhumid conditions with an excellent current density of 1632 mA cm−2. The good miscibility of the components in the membrane worked synergistically to enhance the physical and electrochemical properties.142
The PIL–polymer hybrid membranes can also be used in separation and absorption. Yan and coworkers prepared wastewater treatment media consisting of PIL-grafted polypropylene (PP) nonwoven fabrics by surface-grafting polymerization of IL monomers and photochemical crosslinking. The as-prepared PIL–PP fabrics exhibited excellent switchable oil/water separation (>99%) and dye adsorption performance (410 mg g−1).143 Another type of PIL–polymer composite membrane with sulfonate ionic cross-links and free sulfate groups exhibits both high flux and water selectivity in dehydrating various alcohols due to its strong interpolyelectrolyte binding, higher hydration capability and sorption separation factor.144
4.7 PIL–organic molecule composites
Organic molecules with highly designable structures and abundant electronic, electrical, magnetic, optical, biological, and chemical properties are extensively explored in materials science. The combination of PILs with organic molecules containing an abundance of functional sites may modulate the physical properties of PILs and generate new functional composite materials. One of the major families of PIL–organic molecule composites is a host–guest complex, which employs well-established noncovalent interactions to achieve stimulus-responsive functional materials. For example, Ritter and coworkers reported the pseudo-lower critical solution temperature (LCST) effect of poly(1-vinyl-3-butylimidazolium TFSI) (poly[vbim][TFSI]) based on the solubility change of poly([BVIm][TFSI]) due to supramolecular complexation effects. It was shown that β-CD formed stable complexes with TFSI− at room temperature. At high temperatures, β-CD slipped off from the anion, and the resulting noncomplexed polymer precipitated (Fig. 17). This pseudo-LCST effect of PIL may provide new applications for temperature-sensitive gels.66 Similarly, Yan and coworkers reported shape memory PIL gels with controllable macroscopic swelling via noncovalent host–guest interactions between β-CD and TFSI anions. The quick swelling and shape memory behavior of these PIL gels were observed in a β-CD aqueous solution due to a hydrophobic-to-hydrophilic transition induced by the β-CD/TFSI inclusion complexes; additionally, the PIL gel underwent only slight swelling in the presence of water.145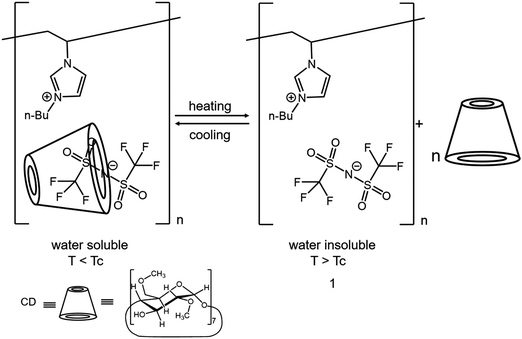 | ||
| Fig. 17 Pseudo-LCST behavior of the poly[vbim][TFSI]–CD composite. Reprinted with permission from ref. 66. Copyright 2008 American Chemical Society. | ||
PILs can also complex with organic acids (aromatic and aliphatic carboxylate acids with carboxylate groups ≥2) based on electrostatic interactions. The high ionic cross-linking density of the system solidifies and stabilizes the ionic network with minimum chain mobility. The generated nanoporous rigid solids show SBET values ranging from 230 to 350 m2 g−1 with tunable pore volumes from 0.63 to 1.54 cm3 g−1. The porous complex composites are more effective in removing methyl orange (MO) dye from an ethanol solution than an activated carbon and mesoporous silica with similar or even higher SBET values; this effect is suggested to stem from the synergistic combination of the polarity, mesopores, ionic character as well as the fine dispersibility of the composite particles in ethanol.63,146 Alternatively, such ionic porous complex composites can form on the surface of Fe3O4, which serves as a magnetic solid phase extraction (MSPE) adsorbent with enhanced adsorption capacity due to the synergistic effect stemming from multiple contributions by the components in the composites, i.e., inclusion, hydrophobicity, π-complexation, and porosity.147
In addition to applications in adsorption, a PIL–organic molecule composite can potentially be used as a sensor. Very recently, we have developed a triazolium PIL–TA (trimesic acid) ionic complex membrane bearing two concurrent structure gradients; the two gradients include an electrostatic complexation (EC) degree gradient and a density distribution gradient for a carbene–NH3 adduct (CNA) along the membrane cross-section (Fig. 18). The two gradients could independently exert a response to organic solvents/compounds and weak acids. In particular, it expressed the highest sensitivity among all soft proton actuators toward acetic acid at the 10−6 mol L−1 M level in aqueous media. It was also capable of discriminating between weak acid species with pKa values of 3–5 by actuation. Moreover, through the competing actuation of the two gradients, it was able to monitor an entire process of chemical reactions that combined multiple steps and carried multiple stimuli with competitive interactions.148
 | ||
| Fig. 18 (a) Schematic illustration of the bending mechanism derived from the tandem gradients of the membrane actuator by external stimuli. (b) Linear correlation of the membrane curvature against CH3COOH concentration. (c) Detection of a broad range of weak acids with different pKa values by a Ptriaz-TA membrane actuator (1 mm × 25 mm × 80 μm). Insets: Photographs of the membrane stripe in different acid solutions. (d) The cascade actuation of the tandem-gradient Ptriaz-TA membrane (1 mm × 25 mm × 120 μm) for monitoring a chemical reaction. (up) Ball-stick models representing the two stimuli driving the cascade actuation. (down) 3D histogram by plotting the conversion of acetic anhydride against the corresponding curvature of the membrane stripe. Reprinted with permission from ref. 148. Copyright 2018 Nature Publishing Group. | ||
4.8 PIL–MOF/POF composites
Metal–organic frameworks (MOFs) and porous organic polymers (POFs) have emerged as a new class of anoporous materials and have been extensively explored in applications including gas storage, separation, catalysis, and sensing. Much effort has been devoted to developing hybridization of MOFs/POFs and polymers with improved functions.149 The polymer hybrid with MOFs/POFs can be achieved either by encapsulation or by surface modification, both of which may lead to intriguing properties. PILs are ionic in nature with highly tunable scaffold structures and these scaffolds are popular for combining with MOFs/POFs to form new composites.Currently, PIL–MOF/POF composites have been explored for catalytic applications, particularly for CO2 cycloaddition reactions. In this case, the counteranions in PILs serve as nucleophilic agents, while the metals in MOF/POF serve as Lewis acid sites that cooperate to catalyze the reaction. For instance, the combination of a porous zinc–porphyrin framework (P-POF-Zn) and PIL can catalyze the cycloaddition reaction of CO2 and propylene oxide, demonstrating a TOF of 433 h−1.150
In view of the flexible nature of linear polymers, their functional moieties have significant movability, thereby exhibiting properties similar to those of their homogeneous counterparts, which makes them promising candidates to be utilized instead of molecular catalysts. The advantage of a nanoporous POF or MOF enables the encapsulated linear PIL to maintain its flexibility; meanwhile, the flexible and mobile catalytic moieties on the linear polymer cooperate with the active sites anchored on the COF pore walls, which is promising for synergistic catalysis. Ma and coworkers deposited linear PILs in close proximity to Lewis acid active sites (Cu) anchored on the wall surfaces of a COF. In addition to the synergistic effect of catalytic sites in the composite, the essential catalytic components enriched in the confined space were beneficial for boosting cooperation and promoting catalytic efficiency toward cycloaddition reactions with atmospheric CO2. This work also afforded an amenable route to bridge natural and artificial systems.151 Similarly, synergy between PILs and porous hosts to enhance CO2 capture and conversion has been observed in Zn–PPh3 integrated porous organic polymers152 or PIL–MIL-101(Cr) composites.153
Such porous hybrids functionalized with ionic species are also useful in selective adsorption and separation. An example was a hierarchically porous structure produced by Guan, Wang and coworkers; they combined a hydrophobic mesoporous PIL copolymer (by copolymerization of amine-functionalized imidazolium-IL monomer and divinylbenzene) with a subsequent modification of ZIF-8 on the surface of a PIL via a facile in situ growth procedure. ZIF-8@PIL showed excellent CO2 adsorption (0.80 mmol g−1 at 298 K) and diffusion (1.50 × 10−3 s−1) properties in comparison to the pure PIL (adsorption capacity: 0.51 mmol g−1 at 298 K, diffusion rate: 1.11 × 10−3 s−1). In particular, small micropores were generated in the growth process due to strong coordination interactions between the amino-alkyl chain on the surfaces of the PIL and ZIF-8, which was beneficial for CO2 storage at low pressure.154 In addition, ZIF-8 nanoparticles and PILs were mixed for the preparation of MMMs for natural gas sweetening and postcombustion CO2 capture.155 Improved gas permeability without much scarifying CO2/N2 and CO2/CH4 selectivity was achieved as compared to their PIL counterparts. Nabais et al. used different MOFs (MIL-53(Al), Cu3(BTC)2 and ZIF-8) as fillers for PIL–MOF MMMs, aiming to maximize the membrane performance toward the purification of syngas. The membrane permeability was considerably influenced by the intrinsic characteristics of the incorporated MOFs, namely, their porous volume, cavity topology and surface area. Among the three different types of MMMs, membranes based on MIL-53(Al) showed the highest improvement in ideal selectivity (up to 13.3), while membranes based on ZIF-8 achieved the highest results for CO2 permeability, up to 97.2 barrer with 30 wt% loading.156
Threading a linear PIL into an MOF was further explored as an ion-exchange material, which is important in a diverse range of applications, including water purification and precious metal recovery. Conventional ion exchange resins, which consist of crosslinked polymers with a low surface area, decrease the contact efficiency between the ion-exchange sites and guest species. In the PIL–MOF composite, the polymer chains are segregated by the ordered MOF structure without entanglement, which forces full contact with solvent molecules that enter the open framework. Consequently, the composite synthesized by the fusion of poly(vinylbenzyltrimethylammonium hydroxide) into ZIF-8 facilitates fast ion exchange behavior toward nitrate (∼50% in 1 min) and gold cyanide (∼100% in 5 min) in solution. In comparison, a conventional ion exchange resin Amberlyst-A26 (nitrate: ∼50% in 60 min, gold cyanide: ∼100% in 30 min) bears unevenly distributed binding sites inside the resin, which are composed of cross-linked chains and a highly irregular porous structure that limits the hydrated domains (Fig. 19).157,158
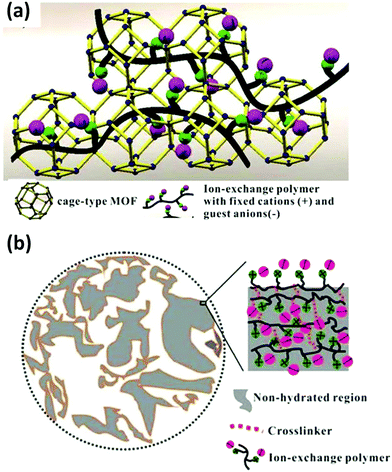 | ||
| Fig. 19 Schematic illustration of the structures of the ion-exchange materials: (a) PVBTAH–ZIF-8 and (b) a microscopic view of a standard ion-exchange resin with random pore distribution, e.g., Amberlyst-A26, with the majority of active sites hidden inside the nonhydrated regions of the bead. Reprinted with permission from ref. 158. Copyright 2014 American Chemical Society. | ||
Composites can not only accelerate adsorption kinetics but also sense toxic gas. Very recently, we developed a morphological replacement method that employed a prefabricated ionically crosslinked porous PIL membrane as a reactive template and precursor. The in situ transformation from the porous PIL membrane bearing a gradient crosslinking density along its cross-section produced an asymmetric nanoporous MOF hybrid membrane that possessed a gradient size and mass distribution of MOF crystals throughout the membrane cross-section (Fig. 20a). The as-synthesized HKUST-1 hybrid membrane, due to its intrinsic asymmetric structure, could actuate at a high speed (bending curvature of 0.42 mm−1 in 0.5 s) when in contact with NH3 gas, which was the first example of MOF membrane-based actuators (Fig. 20b).57 Such a membrane could serve as an NH3 gas sensor as the curvature of the actuator was linearly proportional to the content of NH3 gas.
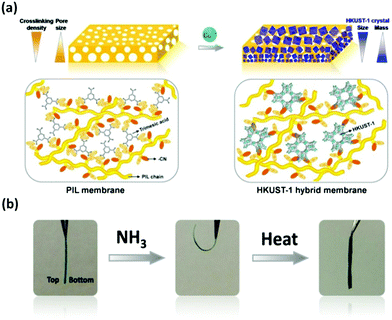 | ||
| Fig. 20 (a) A schematic of the pseudomorphic replacement approach to the preparation of an asymmetric MOF (HKUST-1) hybrid membrane (right) from a porous PIL membrane (left) template and precursor. The process involves the breaking of the electrostatic ionic complexation by the coordination ions and the spontaneous formation of the HKUST-1 phase. Then, it is glued together by the leftover PIL and more locally by the cyano group (red ellipse) of the PIL polymer, which promotes integrity of the macroscopic morphology. (b) Adaptive movement of an HKUST-1 hybrid membrane (11 mm × 2 mm × 38 mm) placed in NH3 gas and then back out after a heat treatment. Reprinted with permission from ref. 57. Copyright 2017 Royal Society of Chemistry. | ||
4.9 PIL–biosubstance composites
A biosubstance is a material intentionally made from substances derived from living (or once-living) organisms. Recently, materials scientists have pursued hybrid or composite biosubstances to synergize the beneficial properties of multiple materials into a superior matrix. The fusion of PILs with biosubstances is an emerging method for fabricating composites with enhanced material performance.159,160 For example, PIL membranes after being impregnated with cellulose nanofibrils had improved tensile strength up to 104 MPa, which is much higher than that of commercial porous polymer filtration membranes.161 Another example is the combination of guar gum (a plant-based polysaccharide) with an imidazolium-based PIL, which leads to a composite with tunable elasticity up to 3 × 104 Pa and a high conductivity of >10−4 S cm−1 at 30 °C; the above results can be attributed to intermolecular polar interactions (mainly hydrogen bonds) and a topologic chain entanglement-induced formation of physical biohybrid ionogels. This medium enables a good compromise between mechanical cohesion and ion transport.162PILs can graft onto the surface of the biosubstance via in situ polymerization to accelerate the conversion of natural biomass into functional porous carbons, modify the surface of the biomass or endow the composites with new functions. For example, Zhang et al. applied PILs to improve the hydrothermal carbonization (HTC) performance of D-fructose and D-glucose to prepare porous nitrogen-doped carbon particles. The carbon products derived from pristine sugars without or with only IL additives showed a small surface area (SBET < 10 m2 g−1); in contrast, the use of PIL additives at weight fractions of 0.55–13.3% significantly increased SBET up to 572 m2 g−1. The better performance of PILs over ILs in the formation of pores was assigned to the multivalent binding power of PILs. The yield of the HTC product from pure fructose was lower than that of HTC with PIL additives, confirming the catalytic effect of PILs in accelerating the HTC process of sugars.163 Similarly, we developed an in situ process to graft a PIL, poly(1-ethyl-3-vinylimidazolium TFSI), onto natural cotton. For this special morphology, the PIL coating could accelerate the activation of cotton into porous carbons even at a weight fraction as low as 3.4 wt%. During the activation process, good preservation of the microscopic fiber structure and the macroscopic morphology was achieved, and the resulting microporous carbon fibers were mechanically flexible. Such a strategy could also be extended to activate sugar-based molecules and polysaccharide biosubstances for porous carbon production.
PILs bearing abundant supramolecular interaction sites are also good for immobilizing enzymes for biocatalysts. For example, a PIL-supported B12 catalyst with a Ru(II) trisbipyridine photosensitizer ([Ru(bpy)3]Cl2) as a visible light-driven photocatalyst (B12-BVIm-Ru polymer) shows enhanced emission and a relatively high quantum yield compared to a monomer mixture in an IL due to the rigidochromic effect of the polymer.164 Goto et al. encapsulated horseradish peroxidase in PIL microparticles (PIL-MP) prepared by polymerization of an IL monomer in a concentrated water-in-oil emulsion. The enzyme encapsulated in PIL-MP was chemically modified with comb-shaped polyethylene glycol-grafted molecules and showed more than twice the level of activity of an enzyme encapsulated in polyacrylamide microparticles.165 Großeheilmann and coworkers reported that a lipase enzyme immobilized in imidazolium-based PIL hydrogels showed significantly increased activity in comparison to a nonimmobilized enzyme. Here, PIL-based hydrogels provided a good environment for lipases due to their high chemical stability and adjustable water content for folding the protein, which was responsible for the improved activity and selectivity of the enzyme. Moreover, the enzyme catalyst was easily recyclable due to the high mechanical and chemical stability of the PILs.166
In addition, PIL–biosubstance composites can be used in other biofields. DNA–polycation complexes are known as one of the most promising systems in gene therapy due to their salient features, such as aqueous solubility, charge neutralization, pH sensitivity, and tunable physicochemical properties. In this regard, Vijayakrishna and coworkers investigated the interaction between calf thymus DNA (ctDNA) and imidazolium-based PILs with three different alkyl side chains (ethyl, butyl, and hexyl). An increase in the binding effect between the PIL and DNA was identified when increasing the length of the alkyl side chain on the PIL, suggesting that there was a binding contribution from both hydrophobic interactions and electrostatic interactions (Fig. 21). However, if the electrostatic cohesion between the polymer and DNA is too strong for the adequate release of DNA into the cell, transfection efficiency can be dramatically suppressed. The ability to manipulate these coulombic interactions is instrumental for the optimization of transfection efficiency.167 Campos and coworkers found that the trisaminocyclopropenium (TAC)-functionalized PIL was highly biocompatible and exhibited efficient DNA transfection. The cyclopropenium cation was stable across a broad pH range, and the resulting materials were particularly robust in utilization.168
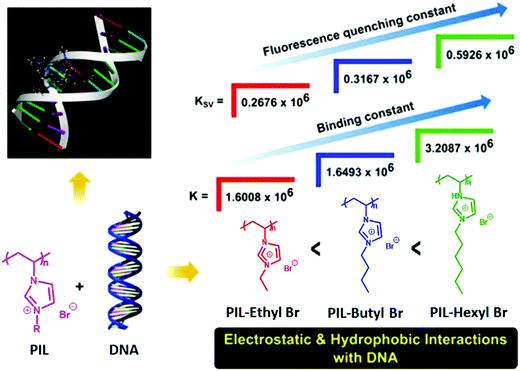 | ||
| Fig. 21 Comparison of binding between DNA and PILs bearing different alkyl side chains. Reprinted with permission from ref. 167. Copyright 2015 American Chemical Society. | ||
4.10 PIL–electrode composites
ILs possessing unique electrochemical characteristics, such as large electrochemical windows and high ion conductivity, have been proposed in several electrochemical applications, including electrochemical capacitors, lithium-ion batteries, fuel cells, and solar batteries. PILs inherit the properties of both ILs and polymer chains. This combination allows a new class of polymeric materials to be engineered as new polymer electrolytes for use in energy, environment, and catalysis applications. In addition, PILs can also be grafted onto traditional electrodes to form new composite electrodes that have an improved accessibility for electrolyte ions at the electrode surface. Randriamahazaka and coworkers deposited a redox-active PIL poly(3-(2-methacryloyloxyethyl)-1-ferrocenylmethylimidazolium TFSI) (poly[MAEImMFc][TFSI]) onto gold electrode surfaces via an SI-ATRP technique. The electrochemical characterization of the PIL-modified electrode in electrolytic acetonitrile solution showed the presence of a reversible redox signal that was characteristic of surface-confined electroactive layers. Ferrocenyl PIL-modified electrodes have been used as reversible redox-responsive materials because the reversible wettability of the polymer can be electrochemically switched on/off. In addition, the redox PILs exhibited electrocatalytic activity and a voltammetric response toward tyrosine.76To date, the development of PILs for electrolyte applications has mainly focused on PILs carrying a TFSI anion because of their highly delocalized charge distribution and excellent plasticizing effect, as well as good compatibility with various electrodes, such as Li metal. To develop better PIL-based solid polymer electrolytes (SPEs), Nie and coworkers prepared an SPE comprised of poly[N,N-dimethyl-N-[2-(methacryloyloxy)ethyl]-N-[2-(2-methoxyethoxy)ethyl]ammonium] (P[C5O2NMA,11]) and LiFSI. It was demonstrated that the ion conductivity of the LiFSI/P[C5O2NMA,11]FSI electrolyte was higher than that of the corresponding TFSI-based electrolyte. The interfacial resistances of the Li symmetric cell (Li metal|polymer electrolytes|Li metal) using the LiFSI/P[C5O2NMA,11]FSI electrolyte were much lower than those using the LiTFSI/P[C5O2NMA,11]TFSI electrolyte. Additionally, the FSI anion displayed a better plasticizing effect than the TFSI anion, which reduced the Tg and increased the ionic conductivity of the polymer electrolytes.169
PILs have also been explored as binders in batteries. Our group has pioneered the use of imidazolium-based PILs for binder applications.170 Paillard and coworkers synthesized cross-linked PIL NPs by dispersion polymerization of 1-vinyl-3-ethylimidazolium TFSI; the above material was used as a binder for Li-ion battery electrodes that were based on graphite microparticles and carbon-coated submicrometric Li4Ti5O12 (LTO) particles. The graphite/PIL NP electrodes were cycled for more than 7 months, and the LTO/PIL NP electrodes provided excellent capacities, rate performances and long-term cycling stability. The excellent binding properties of the nanolatex resulted from the reduction of the PIL at low potentials, leading to chemical bonding of the binder to the carbon surface. Additionally, the use of an insoluble binder strongly reduced electrode cracking or exfoliation from the current collectors, which can be caused by a soluble binder retracting upon drying.171
Similarly, PIL binders possessing different chemical structures, polymer backbones, and polymeric architectures, including linear homopolymers and nanoparticles, have been used as cathode binders in lithium–sulfur batteries. In all cases, the PILs are generally better than the commercial benchmark binder PVDF with respect to cyclability and discharge capacities in extended cycling experiments. The improvement is ascribed to the better compatibility between the PILs and the produced sulfides, which inhibits swelling-induced degradation and retention of the sulfides within the cathode. PIL binders mixed with sulfide species result in more uniformly distributed sulfides in the cathode and better sulfide transport. These features help to mitigate the volume change-induced degradation of electrodes that typically plague Li–S batteries. The uptake of polysulfides by PILs also inhibits the polysulfide shuttling effect during battery cycling, leading to better cycling stability.172
Helms and coworkers highlighted at a molecular level the importance of macromolecular design and mechanistic understanding of PIL binders in the development of Li–S battery technology. PDADMA–TFSI (denoted as PEB-1) was shown to both facilitate Li+ transport through its reconfigurable network of mobile anions and restrict polysulfide diffusion from mesoporous carbon hosts by anion metathesis. Cells carrying such a PIL binder exhibited excellent rate capability (>100 cycles) using cathodes with areal sulfur loadings up to 8.1 mg cm−2. For comparison, composite sulfur cathodes with a PVDF binder exhibited slower and more rapidly degrading electrode kinetics (Fig. 22).173
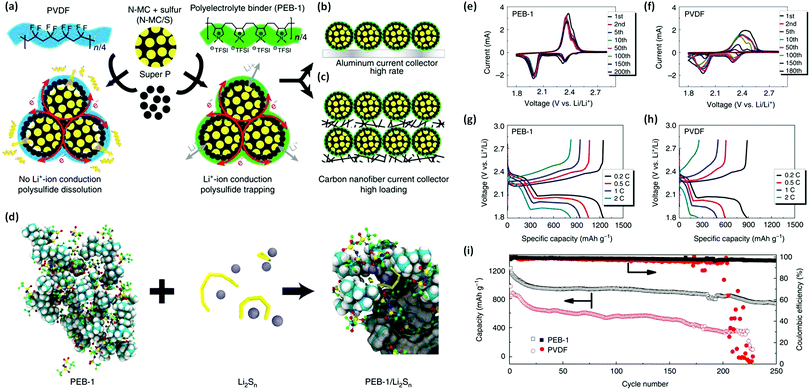 | ||
| Fig. 22 (a) Illustration showing the fabrication of sulfur electrodes with a PVDF or PEB-1 binder. (b) A conventional sulfur cathode and (c) a highly loaded sulfur cathode cast onto an aluminum current collector. (d) Schematic illustrating the formation of complex ion clusters via anion metathesis. (e and f) Long-term cyclic voltammetry (CV). (g and h) Discharge and charge curves at different C rates. (i) Cycling performance for each cell type at a rate of C/5. Reprinted with permission from ref. 173. Copyright 2017 Nature Publishing Group. | ||
4.11 PIL–IL composites
ILs can mix smoothly with PILs to engineer functional PIL–IL composites. One advantage of such composites is their enhanced ionic conductivity in comparison with sole PILs, which are potential candidates as quasi-solid electrolytes in energy conversion and storage devices. PILs in a solid-state normally suffer from limited mobility of the ionic backbone that can be considered to be in a frozen state, and thus only the counteranions serve as mobile charge carriers. The PIL–IL composite may carry the synergy of ILs to increase the number of charge carriers and of PILs for the needed mechanical strength of the electrolyte to reduce the recurring problem of incompatibility between the ILs and other host polymers.174–178 For example, in an imidazolium-based PIL/IL gel system, increasing the free IL content from 50 to 80 wt% produced an ion conductor with conductivity values ≥10−2 S cm−1 at 25 °C and ≈10−1 S cm−1 at 110 °C.176 Oliveira et al. developed a type of gel electrolyte by mixing a PIL matrix and two ILs, namely, 1-methyl-3-butylimidazolium bis(fluorosulfonyl)imide (MBIMFSI) and 1,2-dimethyl-3-propylimidazolium TFSI (DPITFSI), as a quasi-solid-state electrolyte for use in supercapacitors.178 The synthesized PIL/IL gel electrolyte exhibited an ion conductivity as high as 3.2 × 10−3 S cm−1 at 25 °C and an electrochemical window of 2.8 V (obtained by linear sweep voltammetry). The corresponding supercapacitor devices showed a specific capacitance of 49.1 F g−1 and 41.2 F g−1 at the positive and negative electrodes, respectively, as well as a satisfactory energy density (9.8 W h kg−1) and power density (532 W kg−1) at 0.5 A g−1 and 2.5 V. On the basis of these achievements, by a judicious choice of the PIL matrix and suitable ILs as well as the optimized composite fabrication method, PIL/IL composites may play a more crucial role in the current pursuit of high-safety energy conversion and storage devices.In addition to being used as quasi-solid electrolytes, PIL–IL composites have great potential to serve as MMMs for gas separation, e.g., CO2/N2 or CO2/CH4. Despite the fact that PILs provide an alternative choice to design IL-based membrane materials for CO2 separation, the relatively low gas permeability and diffusivity of neat PIL membranes forces researchers instead to develop PIL/IL composite membranes. The aim is to combine their particular features together for enhanced CO2 transport properties.179–183 In a PIL/IL composite membrane, the PIL provides the necessary mechanical stability while the IL secures a high gas permeability and diffusivity. Noble and Gin et al. have carried out pioneering work in this area (Fig. 23). The proof-of-concept for PIL/IL composite membranes for gas separation was reported in 2008.184 Over the past few years, their systematic studies include the preparation of PIL/IL composites and their use as high-performance, ultrathin selective layers in composite membranes for CO2/N2 separation. For example, 100-nm-thick active layers were produced for high-performance composite membranes with CO2 permeance ≥6000 GPU (gas permeation units) and an ideal CO2/N2 selectivity of approximately 22. The detailed work has been summarized in a recent and brief review by Noble, Gin and coworkers.179 In fact, the addition of free ILs increases the CO2 permeability of PIL/IL membranes, and the gas permeation properties of the free IL significantly influence the CO2 separation performance of the composites. Consequently, the choice of an appropriate IL is crucial in tailoring the PIL/IL membrane performance in CO2 separation. Marrucho,183 Chung,185 and other researchers have also contributed significantly to this area, which can be referred to in the recent comprehensive review article.182
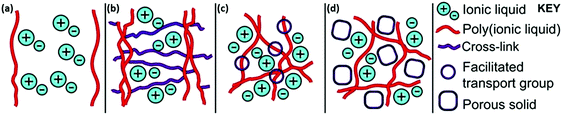 | ||
| Fig. 23 A series of PIL/IL ion-gel membranes were developed by Noble and Gin et al. (a) A linear PIL/IL ion-gel system in a composite membrane; (b) cross-linked PIL/IL ion-gel materials with improved CO2 permeability; (c) PIL/IL ion-gels that incorporate facilitated transport groups to improve CO2 permeability and CO2/N2 separation selectivity; and (d) MMMs that incorporate a microporous solid with improved CO2 permeability and CO2/light gas selectivity. Reprinted with permission from ref. 179. Copyright 2016 American Chemical Society. | ||
In addition to the above-mentioned extensively explored methods for CO2 separation from flue or other similar gases, PIL/IL composite membranes can also be used for CO2/H2 separation. In particular, the implementation of high-performance and cost-effective biohydrogen (bioH2) purification techniques is of vital importance, especially for fuel cell applications.186,187 Carlisle et al. explored CO2/H2 separation through imidazolium-based PIL/IL gel membranes produced by UV-initiated polymerization.186 The time-lag experiments performed at room temperature and 200 kPa of feed pressure showed an ideal CO2/H2 selectivity ranging from 6.6 to 12 for membranes prepared with 5 to 100 mol% of a cross-linking monomer and different amounts of an IL monomer. Their optimized composite system, containing 100 mol% cross-linking monomer and 75 wt% IL, showed a CO2 permeability of 540 barrer and a CO2/H2 selectivity of 12. Marrucho's group used pyrrolidinium-based PILs with [C(CN)3]− or [NTf2]− anions and different amounts of ILs ([C2mim][C(CN)3], [C4mpyr][NTf2] or [C2mim][NTf2]) incorporated into the CO2/H2 separation membranes. Particularly at 308 K, the PIL C(CN)3–60 IL C(CN)3 composite membrane delivered a CO2 permeability of 505 barrer and a CO2/H2 selectivity of 12.5.188
4.12 PIL–other composites
In addition to the PIL-based composites mentioned above, PILs have been explored in combination with other materials to produce composites with intriguing structures and improved performance in catalysis, separation, and sensing.PILs were shown to be important elements for fabricating membranes as separators and sensors. For example, via solvothermal copolymerization of an IL monomer 1-ethyl-3-vinylimidazolium bromide (EVImBr) and divinylbenzene (DVB) inside a tissue paper as a template, a polymer-paper hybrid membrane bearing high porosity and mechanical flexibility was fabricated.9 The hybrid membrane is porous, and the surface area is dependent on the weight fraction of the copolymer impregnated inside the tissue paper. The as-synthesized composite membrane shows controlled surface wettability in terms of ethanol wetting and vacuum drying, endowing the membrane with a switchable oil/water separation function.
When combined with biological templates, PILs were able to replicate and preserve complicated and inherent structural motifs of the templates at a micro- to macroscale due to the advantages of their high surface activity, their reaction with the carbohydrate template and their resulting oxidative stability. We functionalized Hoplia coerulea beetles with a PIL layer on their surface, followed by a low temperature calcination under nitrogen to maintain the surface morphology at the microscale.189 In this process, the thin PIL coating layer bearing a dicyanamide (DCA) anion provided precise microstructure preservation. The DCA anions built up cross-linking points at elevated temperature, thus stabilizing the scale morphology since DCA is known to undergo a trimerization-type crosslinking reaction, which forms triazine networks and reduces the structural fragmentation at elevated temperature.
Structurally well-defined and tailored PILs provide the ability to reach a low Tg and promote a high chain mobility, which increase ion conductivity. However, the weak intermolecular interactions of PILs result in poor mechanical cohesion, limiting their applicability. To solve this problem, Walther and coworkers combined the mechanical design principles of nacre-mimetics with the ion conductive nature of a PIL, poly(1-ethyl-3-vinylimidazolium dicyanamide), resulting in nanoplatelets bearing a core–shell structure.190 PILs were adsorbed onto the anionic basal planes of the nanoclay due to electrostatic complexation as well as hydrophobic and van der Waals interactions. The as-prepared nanocomposites showed attractive multifunctional property profiles with high transparency, satisfactory values of ion conductivity, and high stiffness and strength.
5. Conclusion and perspective
Upon the ongoing exploration of new PIL chemical structures, PIL-based materials chemistry has entered a very exciting era. Chemists now have expanded beyond the synthesis and structural characterization of new PILs, and more efforts will be made to integrate PILs into useful materials that tackle problems and challenges in life science, energy, environment, etc.This review article has described the synthetic methodologies for PIL composites, as well as the fascinating properties of the hybrid materials. The integration of PILs into different substances has provided unique aspects in composites, especially the synergistic effects that arise from each component. Nevertheless, the development of PIL composites is still in its infancy. Although some structures have been synthesized, dedicated integration of PILs with more functions is highly desirable for further development in terms of materials chemistry. For example, Mecerreye and coworkers recently incorporated organic redox moieties such as nitroxide 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or anthraquinone (AQ) derivatives as counteranions into PDADMA-type PILs. This new family of redox-active PILs can be applied in several electrochemical energy storage technologies, such as lithium batteries, as electrocatalysts in fuel cells and metal–air batteries or as electrolytes in organic redox flow batteries (Fig. 24a).191 Another example is the introduction of chiral moieties into PILs, which can be used as fluorescence off/on sensors for chiral recognition or asymmetric catalytic applications (Fig. 24b).192,193 These emerging examples highlight a promising way to engineer diverse functional PILs (e.g., conductivity) for composite fabrication and applications.
 | ||
| Fig. 24 A selection of emerging and new functional PIL candidates for composite preparation. Reprinted with permission from ref. 191–193. Copyright 2017 Royal Society of Chemistry and 2018 American Chemical Society. | ||
Currently, most of the studies on PIL composites focus on metal nanostructured materials or carbons, and the resultant composites have already shown enhanced electric, thermal and mechanical properties, which outperform traditional materials. These advantages arise from the periodic arrangement of ILs, and the ion conductivity, electrochemical stability and processability; furthermore, the high compatibility of PILs with substances based on multiple interactions and synergetic effects also provide advantages. However, there is considerable room to explore new functions and materials, especially using PILs in combination with emerging nanoporous materials such as MOFs, COFs, or bio-substances. In this regard, intriguing nanostructured composites with hierarchical architectures and fantastic properties, such as host–guest synergistic functions and heterogeneous biocatalysis with high efficiency, can be expected and represent an exciting area of great opportunity.
Further investigation of novel PIL composite materials is also closely dependent on a better understanding of structure–property relationships and physicochemical behaviors. In most reported PIL composites, an active interplay between the PIL and the other substance can be identified. A better understanding of the mechanism, especially the heterojunction effect derived from a biphase between PIL and substance, will undoubtedly help judicious design and improve the value of the composite materials. This knowledge will inspire ideas and catalyze more directions to harness the PIL family to a wider scope of composites.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
J. K. S. acknowledges Starting Grant from Beijing Institute of Technology (3100011181910). J. Y. is grateful for financial support from the ERC Starting Grant NAPOLI-639720 from European Research Council, Stockholm University Strategic fund, Swedish Research Council grant 2018-05351, Dozentenpreis 15126 from Verband der Chemischen Industrie e.V. (VCI) in Germany, and the Wallenberg Academy Fellow program (KAW 2017.0166) in Sweden. Q. Z. acknowledges the funding from National Natural Science Foundation of China (Grant No. 21703172) and the Fundamental Research Funds for the Central Universities (Grant No. 310201911cx043).References
- J. Yuan and M. Antonietti, Polymer, 2011, 52, 1469–1482 CrossRef CAS.
- J. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009–1036 CrossRef CAS.
- J. Lu, F. Yan and J. Texter, Prog. Polym. Sci., 2009, 34, 431–448 CrossRef CAS.
- D. Mecerreyes, Prog. Polym. Sci., 2011, 36, 1629–1648 CrossRef CAS.
- W. Qian, J. Texter and F. Yan, Chem. Soc. Rev., 2017, 46, 1124–1159 RSC.
- J. Gong, H. Lin, J. W. C. Dunlop and J. Yuan, Adv. Mater., 2017, 29, 1605103 CrossRef PubMed.
- P. Kallem, A. Eguizabal, R. Mallada and M. P. Pina, ACS Appl. Mater. Interfaces, 2016, 8, 35377–35389 CrossRef CAS PubMed.
- P. Zhang, M. Li, B. Yang, Y. Fang, X. Jiang, G. M. Veith, X. G. Sun and S. Dai, Adv. Mater., 2015, 27, 8088–8094 CrossRef CAS PubMed.
- C. X. Cao, J. Yuan, J. P. Cheng and B. H. Han, Sci. Rep., 2017, 7, 3101 CrossRef PubMed.
- R. Lambert, A. L. Wirotius, J. Vignolle and D. Taton, Polym. Chem., 2019, 10, 460–466 RSC.
- R. Lambert, A. L. Wirotius, S. Garmendia, P. Berto, J. Vignolle and D. Taton, Polym. Chem., 2018, 9, 3199–3204 RSC.
- S. Chen, Y. Xiang, M. K. Banks, C. Peng, W. Xu and R. Wu, Nanoscale, 2018, 10, 20043–20052 RSC.
- W. Bi, M. Tian and K. H. Row, Analyst, 2012, 137, 2017–2020 RSC.
- K. Täuber, Q. Zhao, M. Antonietti and J. Yuan, ACS Macro Lett., 2015, 4, 139–142 Search PubMed.
- H. Du, X. Liu, Y. Yu, Y. Xu, Y. Wang and Z. Liang, Macromol. Chem. Phys., 2016, 217, 2626–2634 CrossRef CAS.
- Z. Li, W. Wang, Y. Chen, C. Xiong, G. He, Y. Cao, H. Wu, M. D. Guiver and Z. Jiang, J. Mater. Chem. A, 2016, 4, 2340–2348 RSC.
- J. Gong, M. Antonietti and J. Yuan, Angew. Chem., Int. Ed., 2017, 56, 7557–7563 CrossRef CAS PubMed.
- D. Ager, V. A. Vasantha, R. Crombez and J. Texter, ACS Nano, 2014, 8, 11191–11205 CrossRef CAS PubMed.
- I. K. Oh, J. H. Jung, J. H. Jeon and S. Vadahanambi, Smart Mater. Struct., 2010, 19, 075009 CrossRef.
- G. Choi, G. M. Jeong, M. S. Oh, M. Joo, S. G. Im, K. J. Jeong and E. Lee, ACS Biomater. Sci. Eng., 2018, 4, 2614–2622 CrossRef CAS.
- M. G. Cowan, D. L. Gin and R. D. Noble, Acc. Chem. Res., 2016, 49, 724–732 CrossRef CAS PubMed.
- R. Gao, D. Wang, J. R. Heflin and T. E. Long, J. Mater. Chem., 2012, 22, 13473–13476 RSC.
- M. H. Yang, B. G. Choi, S. C. Jung, Y. K. Han, Y. S. Huh and S. B. Lee, Adv. Funct. Mater., 2014, 24, 7301–7309 CrossRef CAS.
- T. T. Tung, C. Pham-Huu, I. Janowska, T. Y. Kim, M. Castro and J. F. Feller, Small, 2015, 11, 3485–3493 CrossRef CAS PubMed.
- C. P. Lee and K. C. Ho, Eur. Polym. J., 2018, 108, 420–428 CrossRef CAS.
- R. Marcilla, J. A. Blazquez, J. Rodriguez, J. A. Pomposo and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 208–212 CrossRef CAS.
- Y. Ye and Y. A. Elabd, Macromolecules, 2011, 44, 8494–8503 CrossRef CAS.
- J. Pinaud, J. Vignolle, Y. Gnanou and D. Taton, Macromolecules, 2011, 44, 1900–1908 CrossRef CAS.
- C. Huang, X. Qian and R. Yang, Mater. Sci. Eng., R, 2018, 132, 1–22 CrossRef.
- A. Shaplov, D. Ponkratov and Y. Vygodskii, Polym. Sci., Ser. B, 2016, 58, 73–142 CrossRef CAS.
- Y. Ye and Y. A. Elabd, Polymer, 2011, 52, 1309–1317 CrossRef CAS.
- R. Marcilla, J. A. Blazquez, R. Fernandez, H. Grande, J. A. Pomposo and D. Mecerreyes, Macromol. Chem. Phys., 2005, 206, 299–304 CrossRef CAS.
- J. Yuan, S. Prescher, K. Sakaushi and M. Antonietti, J. Mater. Chem. A, 2015, 3, 7229–7234 RSC.
- H. Ohno, Design of ion conductive polymers based on ionic liquids, Macromol. Symp., 2007, 551–556 CrossRef CAS.
- W. Ogihara, S. Washiro, H. Nakajima and H. Ohno, Electrochim. Acta, 2006, 51, 2614–2619 CrossRef CAS.
- M. Yoshizawa and H. Ohno, Chem. Lett., 1999, 889–890 CrossRef CAS.
- H. Chen, J.-H. Choi, D. Salas-de la Cruz, K. I. Winey and Y. A. Elabd, Macromolecules, 2009, 42, 4809–4816 CrossRef CAS.
- H. Hu, W. Yuan, L. Lu, H. Zhao, Z. Jia and G. L. Baker, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2104–2110 CrossRef CAS.
- S. Washiro, M. Yoshizawa, H. Nakajima and H. Ohno, Polymer, 2004, 45, 1577–1582 CrossRef CAS.
- R. L. Weber, Y. Ye, S. M. Banik, Y. A. Elabd, M. A. Hickner and M. K. Mahanthappa, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1287–1296 CrossRef CAS.
- G. Appetecchi, G.-T. Kim, M. Montanino, M. Carewska, R. Marcilla, D. Mecerreyes and I. De Meatza, J. Power Sources, 2010, 195, 3668–3675 CrossRef CAS.
- M. Hayyan, F. S. Mjalli, M. A. Hashim, I. M. AlNashef and T. X. Mei, J. Ind. Eng. Chem., 2013, 19, 106–112 CrossRef CAS.
- G. G. Eshetu, D. Mecerreyes, M. Forsyth, H. Zhang and M. Armand, Mol. Syst. Des. Eng., 2019, 4, 294–309 RSC.
- A. L. Pont, R. Marcilla, I. De Meatza, H. Grande and D. Mecerreyes, J. Power Sources, 2009, 188, 558–563 CrossRef CAS.
- R. Marcilla, M. L. Curri, P. D. Cozzoli, M. T. Martínez, I. Loinaz, H. Grande, J. A. Pomposo and D. Mecerreyes, Small, 2006, 2, 507–512 CrossRef CAS PubMed.
- M. Antonietti, Y. Shen, T. Nakanishi, M. Manuelian, R. Campbell, L. Gwee, Y. A. Elabd, N. Tambe, R. Crombez and J. Texter, ACS Appl. Mater. Interfaces, 2010, 23, 649–653 CrossRef PubMed.
- J. Texter, R. Crombez, R. Maniglia, X. Ma, V. Arjunan, V. M. Manuelian, R. Campbell, L. Slater and T. Mourey, J. Surfactants Deterg., 2019, 22, 1059–1071 CAS.
- R. Marcilla, E. Ochoteco, C. Pozo-Gonzalo, H. Grande, J. A. Pomposo and D. Mecerreyes, Macromol. Rapid Commun., 2005, 26, 1122–1126 CrossRef CAS.
- T. Kim, M. Suh, S. J. Kwon, T. H. Lee, J. E. Kim, Y. J. Lee, J. H. Kim, M. Hong and K. S. Suh, Macromol. Rapid Commun., 2009, 30, 1477–1482 CrossRef CAS PubMed.
- T. Kim, H. Lee, J. E. Kim and K. S. Suh, ACS Nano, 2010, 4, 1612–1618 CrossRef CAS PubMed.
- T. Y. Kim, H. W. Lee, M. Stoller, D. R. Dreyer, C. W. Bielawski, R. S. Ruoff and K. S. Suh, ACS Nano, 2011, 5, 436–442 CrossRef CAS PubMed.
- J. K. Sun, M. Antonietti and J. Yuan, Chem. Soc. Rev., 2016, 45, 6627–6656 RSC.
- X. Li, K. Wang, N. Ma and X. Jia, Front. Chem., 2017, 5, 69 CrossRef PubMed.
- M. R. Gao, S. H. Yu, J. Yuan, W. Zhang and M. Antonietti, Angew. Chem., Int. Ed., 2016, 55, 12812–12816 CrossRef CAS PubMed.
- J. K. Sun, Z. Kochovski, W. Zhang, H. Kirmse, Y. Lu, M. Antonietti and J. Yuan, J. Am. Chem. Soc., 2017, 139, 8971–8976 CrossRef CAS PubMed.
- X. He, W. Yang and X. Pei, Macromolecules, 2008, 411, 34615–34621 Search PubMed.
- J. K. Sun, H. J. Lin, W. Y. Zhang, M. R. Gao, M. Antonietti and J. Yuan, Mater. Horiz., 2017, 4, 681–687 RSC.
- K. Ahmed, A. Khosla and H. Furukawa, Nanosensors, Biosensors, Info-tech Sensors & 3d Systems, 2017 Search PubMed.
- L. Y. Chang, C. P. Lee, C. T. Li, M. H. Yeh, K. C. Ho and J. J. Lin, J. Mater. Chem. A, 2014, 2, 20814–20822 RSC.
- Z. Zheng, J. Guo, H. Mao, Q. Xu, J. Qin and F. Yan, ACS Biomater. Sci. Eng., 2017, 3, 922–928 CrossRef CAS.
- H. Li, Q. Zhang, X. Liu, F. Chang, Y. Zhang, W. Xue and S. Yang, Bioresour. Technol., 2013, 144, 21–27 CrossRef CAS PubMed.
- R. N. Biboum, F. Doungmene, B. Keita, P. D. Oliveira, L. Nadjo, B. Lepoittevin, P. Roger, F. Brisset, P. Mialane and A. Dolbecq, J. Mater. Chem., 2011, 22, 319–323 RSC.
- Q. Zhao, S. Soll, M. Antonietti and J. Yuan, Polym. Chem., 2013, 4, 2432–2435 RSC.
- Q. Zhao, M. Yin, A. P. Zhang, S. Prescher, M. Antonietti and J. Yuan, J. Am. Chem. Soc., 2013, 135, 155549 Search PubMed.
- B. Zhang, G. Sudre, G. Quintard, A. Serghei, L. David, J. Bernard, E. Fleury and A. Charlot, Carbohydr. Polym., 2017, 157, 586–595 CrossRef CAS PubMed.
- S. Amajjahe and H. Ritter, Macromolecules, 2008, 41, 3250–3253 CrossRef CAS.
- A. Panniello, C. Ingrosso, P. Coupillaud, M. Tamborra and M. Striccoli, Materials, 2014, 7, 591–610 CrossRef CAS PubMed.
- X. Li, Z. Zhang, S. Li, K. Yang and L. Yang, J. Mater. Chem. A, 2017, 5, 21362–21369 RSC.
- M. Brinkkötter, E. I. Lozinskaya, D. O. Ponkratov, P. S. Vlasov, M. P. Rosenwinkel, I. A. Malyshkina, Y. Vygodskii, A. S. Shaplov and M. Schönhoff, Electrochim. Acta, 2017, 237, 237–247 CrossRef.
- L. C. Tomé, D. Mecerreyes, C. S. R. Freire, L. P. N. Rebelo and I. M. Marrucho, J. Mater. Chem. A, 2014, 2, 5631–5639 RSC.
- J. F. Olorunyomi, K. Y. Chan, L. Gao, A. Voskanyan and C. Y. V. Li, Microporous Mesoporous Mater., 2018, 259, 255–263 CrossRef CAS.
- Q. Sun, Y. Tang, B. Aguila, S. Wang, F. S. Xiao, P. K. Thallapally, A. M. Al-Enizi, A. Nafady and S. Ma, Angew. Chem., Int. Ed., 2019, 58, 8670–8675 CrossRef CAS PubMed.
- D. Zhou, R. Liu, J. Zhang, X. Qi, Y. B. He, B. Li, Q. H. Yang, Y. S. Hu and F. Kang, Nano Energy, 2017, 33, 45–54 CrossRef CAS.
- Y. Jiang, J. L. Freyer, P. Cotanda, S. D. Brucks, K. L. Killops, J. S. Bandar, C. Torsitano, N. P. Balsara, T. H. Lambert and L. M. Campos, Nat. Commun., 2015, 6, 5950 CrossRef PubMed.
- R. Luo, Y. Chen, Q. He, X. Lin, Q. Xu, X. He, W. Zhang, X. Zhou and H. Ji, ChemSusChem, 2017, 10, 1526–1533 CrossRef CAS PubMed.
- V. Bui-Thi-Tuyet, G. Trippé-Allard, J. Ghilane and H. Randriamahazaka, ACS Appl. Mater. Interfaces, 2016, 8, 28316–28324 CrossRef CAS PubMed.
- X. He, Y. Wu and X. Pei, Macromolecules, 2008, 41, 4615–4621 CrossRef CAS.
- W. Jia, Y. Wu, H. Jing, A. Qi, X. Dan, Y. Wu, F. Li and G. Li, J. Mater. Chem., 2010, 20, 8617–8623 RSC.
- P. Wang, Y. N. Zhou, J. S. Luo and Z. H. Luo, Polym. Chem., 2013, 5, 882–891 RSC.
- C. Hua, W. Ping, J. Luo, J. Fransaer, D. E. D. Vos and Z. H. Luo, Ind. Eng. Chem. Res., 2015, 54, 3107–3115 CrossRef.
- J. W. Liu, M. M. Wang, Y. Zhang, L. Han, X. W. Chen and J. H. Wang, RSC Adv., 2014, 4, 61936–61943 RSC.
- J. Liu, Y. Liang, J. Shen and Q. Bai, Anal. Bioanal. Chem., 2018, 410, 573–584 CrossRef CAS PubMed.
- L. Mao, Y. Li, C. Chi, H. SzeOn Chan and J. Wu, Nano Energy, 2014, 6, 119–128 CrossRef CAS.
- R. Zhang, S. Ji, N. Wang, L. Wang, G. Zhang and J. R. Li, Angew. Chem., Int. Ed., 2014, 53, 9775–9779 CrossRef CAS PubMed.
- X. Mu, J. Meng, Z. C. Li and Y. Kou, J. Am. Chem. Soc., 2005, 127, 9694–9695 CrossRef CAS PubMed.
- A. Dani, V. Crocellà, L. Maddalena, C. Barolo and E. Groppo, J. Phys. Chem. C, 2016, 120, 1683–1692 CrossRef CAS.
- S. Doherty, J. G. Knight, T. Backhouse, E. Abood, H. Al-Shaikh, A. R. Clemmet, J. R. Ellison, R. A. Bourne, T. W. Chamberlain and R. Stones, Adv. Synth. Catal., 2018, 360, 3716–3731 CrossRef CAS.
- Y. Yang, M. Ambrogi, H. Kirmse, Y. Men, M. Antonietti and J. Yuan, Chem. Mater., 2015, 27, 127–132 CrossRef CAS.
- P. Zhang, Z. A. Qiao, X. Jiang, G. M. Veith and S. Dai, Nano Lett., 2015, 15, 823–828 CrossRef CAS PubMed.
- H. Fang, S. Sun, P. Liao, Y. Hu and J. Zhang, J. Mater. Chem. A, 2018, 6, 2115–2121 RSC.
- S. Montolio, C. Vicent, V. Aseyev, I. Alfonso, M. Isabel Burguete, H. Tenhu, E. García-Verdugo and S. V. Luis, ACS Catal., 2016, 6, 7230–7237 CrossRef CAS.
- S. Prescher, F. Polzer, Y. Yang, M. Siebenbürger, M. Ballauff and J. Yuan, J. Am. Chem. Soc., 2014, 136, 12–15 CrossRef CAS PubMed.
- K. T. Prabhu Charan, N. Pothanagandhi, K. Vijayakrishna, A. Sivaramakrishna, D. Mecerreyes and B. Sreedhar, Eur. Polym. J., 2014, 60, 114–122 CrossRef CAS.
- S. Ghasemi and Z. A. Harandi, RSC Adv., 2018, 8, 14570–14578 RSC.
- D. Batra, S. Seifert, L. M. Varela, A. C. Y. Liu and M. A. Firestone, Adv. Funct. Mater., 2007, 17, 1279–1287 CrossRef CAS.
- Y. Lu, H. Zhu, W.-J. Wang, B. G. Li and S. Zhu, ACS Sustainable Chem. Eng., 2017, 5, 2829–2835 CrossRef CAS.
- R. Zhang, P. Su, L. Yang and Y. Yang, J. Sep. Sci., 2014, 37, 1503–1510 CrossRef CAS PubMed.
- L. Yang, P. Su, X. Chen, R. Zhang and Y. Yang, Anal. Methods, 2015, 7, 3246–3252 RSC.
- H. Han, T. Jiang, T. Wu, D. Yang and B. Han, ChemCatChem, 2015, 7, 3526–3532 CrossRef CAS.
- L. Zhang, J. Du, T. Ran, H. Gao and Y. Liao, J. Mater. Sci., 2016, 51, 7186–7198 CrossRef CAS.
- X. Chen, Q. Li, J. Zhao, L. Qiu, Y. Zhang, B. Sun and F. Yan, J. Power Sources, 2012, 207, 216–221 CrossRef CAS.
- C. Willa, J. Yuan, M. Niederberger and D. Koziej, Adv. Funct. Mater., 2015, 25, 2537–2542 CrossRef CAS.
- C. Wang, X. Ye, Z. Wang, T. Wu, Y. Wang and C. Li, Anal. Chem., 2017, 89, 12391–12398 CrossRef CAS PubMed.
- B. Wu, D. Hu, Y. Kuang, B. Liu, X. Zhang and J. Chen, Angew. Chem., Int. Ed., 2009, 48, 4751–4754 CrossRef CAS PubMed.
- Y. Ding, A. Klyushin, X. Huang, T. Jones, D. Teschner, F. Girgsdies, T. Rodenas, R. Schlögl and S. Heumann, Angew. Chem., Int. Ed., 2018, 57, 3514–3518 CrossRef CAS PubMed.
- N. Patil, M. Aqil, A. Aqil, F. Ouhib, R. Marcilla, A. Minoia, R. Lazzaroni, C. Jerôme and C. Detrembleur, Chem. Mater., 2018, 30, 5831–5835 CrossRef CAS.
- K. Ahmed, A. Khosla, M. Kawakami and H. Furukawa, Nanosensors, Biosensors, Info-tech Sensors & 3d Systems, 2017 Search PubMed.
- D. Gendron, A. Ansaldo, G. Bubak, L. Ceseracciu, G. Vamvounis and D. Ricci, Compos. Sci. Technol., 2015, 117, 364–370 CrossRef CAS.
- H. Lin, J. Gong, M. Eder, R. Schuetz, H. Peng, J. W. C. Dunlop and J. Yuan, Adv. Mater. Interfaces, 2017, 4, 1600768 CrossRef.
- J. Li, Q. Li, Y. Zeng, T. Tang, Y. Pan and L. Li, RSC Adv., 2015, 5, 717–725 RSC.
- H. Mao, J. Liang, H. Zhang, Q. Pei, D. Liu, S. Wu, Y. Zhang and X. M. Song, Biosens. Bioelectron., 2015, 70, 289–298 CrossRef CAS PubMed.
- H. Kazerooni and B. Nassernejad, RSC Adv., 2014, 4, 34604–34609 RSC.
- L. Cseri, J. Baugh, A. Alabi, A. AlHajaj, L. Zou, R. A. W. Dryfe, P. M. Budd and G. Szekely, J. Mater. Chem. A, 2018, 6, 24728–24739 RSC.
- H. He, S. Averick, E. Roth, D. Luebke, H. Nulwala and K. Matyjaszewski, Polymer, 2014, 55, 3330–3338 CrossRef CAS.
- S. Wang, Q. X. Shi, Y. S. Ye, Y. Xue, Y. Wang, H. Y. Peng, X. L. Xie and Y. W. Mai, Nano Energy, 2017, 33, 110–123 CrossRef CAS.
- X. Li, S. Li, Z. Zhang, J. Huang, L. Yang and S. Hirano, J. Mater. Chem. A, 2016, 4, 13822–13829 RSC.
- P. Wang, Y. N. Zhou, J. S. Luo and Z. H. Luo, Polym. Chem., 2014, 5, 882–891 RSC.
- H. Cheng, P. Wang, J. Luo, J. Fransaer, D. E. De Vos and Z. H. Luo, Ind. Eng. Chem. Res., 2015, 54, 3107–3115 CrossRef CAS.
- H. Qiu, A. K. Mallik, M. Takafuji, S. Jiang and H. Ihara, Analyst, 2012, 137, 2553–2555 RSC.
- S. Badragheh, M. Zee and M. R. T. B. Olyai, RSC Adv., 2018, 8, 30550–30561 RSC.
- W. Hou, Q. Wang, Z. Guo, J. Li, Y. Zhou and J. Wang, Catal. Sci. Technol., 2017, 7, 1006–1016 RSC.
- G. Zarca, W. J. Horne, I. Ortiz, A. Urtiaga and J. E. Bara, J. Membr. Sci., 2016, 515, 109–114 CrossRef CAS.
- X. Sui, M. A. Hempenius and G. J. Vancso, J. Am. Chem. Soc., 2012, 134, 4023–4025 CrossRef CAS PubMed.
- X. Feng, K. Zhang, P. Chen, X. Sui, M. A. Hempenius, B. Liedberg and G. J. Vancso, Macromol. Rapid Commun., 2016, 37, 1939–1944 CrossRef CAS PubMed.
- K. Zhang, X. Feng, X. Sui, M. A. Hempenius and G. J. Vancso, Angew. Chem., Int. Ed., 2014, 53, 13789–13793 CrossRef CAS PubMed.
- X. Feng, X. Sui, M. A. Hempenius and G. J. Vancso, J. Am. Chem. Soc., 2014, 136, 7865–7868 CrossRef CAS PubMed.
- K. Zhang, M. Zhang, X. Feng, M. A. Hempenius and G. J. Vancso, Adv. Funct. Mater., 2017, 27, 1702784 CrossRef.
- C. Yuan, J. Guo, M. Tan, M. Guo, L. Qiu and F. Yan, ACS Macro Lett., 2014, 3, 271–275 CrossRef CAS.
- J. Guo, C. Yuan, M. Guo, L. Wang and F. Yan, Chem. Sci., 2014, 5, 3261–3266 RSC.
- S. Doherty, J. G. Knight, M. A. Carroll, A. R. Clemmet, J. R. Ellison, T. Backhouse, N. Holmes, L. A. Thompson and R. A. Bourne, RSC Adv., 2016, 6, 73118 RSC.
- C. Gao, G. Chen, X. Wang, J. Li, Y. Zhou and J. Wang, Chem. Commun., 2015, 51, 4969–4972 RSC.
- P. Zhao, Y. Leng and J. Wang, Chem. Eng. J., 2012, 204–206, 72–78 CrossRef CAS.
- G. Chen, W. Hou, J. Li, X. Wang, Y. Zhou and J. Wang, Dalton Trans., 2016, 45, 4504–4508 RSC.
- Y. Leng, J. Wu, P. Jiang and J. Wang, Catal. Sci. Technol., 2014, 4, 1293–1300 RSC.
- Y. Leng, J. Liu, P. Jiang and J. Wang, ACS Sustainable Chem. Eng., 2015, 3, 170–176 CrossRef CAS.
- N. N. Rozik and S. L. Abd El-Messieh, Polym. Bull., 2017, 74, 3595–3604 CrossRef CAS.
- M. Tokuda, T. Shindo and H. Minami, Langmuir, 2013, 29, 11284–11289 CrossRef CAS PubMed.
- C. Zheng, Y. Liu, Y. Dong, F. He, X. Zhao and J. Yin, Macromol. Rapid Commun., 2018, 1800351 Search PubMed.
- Q. Zhao, P. Zhang, M. Antonietti and J. Yuan, J. Am. Chem. Soc., 2012, 134, 11852–11855 CrossRef CAS PubMed.
- V. K. Tiwari, Y. Lee, G. Song, K. L. Kim, Y. J. Park and C. Park, J. Polym. Sci., Part B: Polym. Phys., 2018, 56, 795–802 CrossRef CAS.
- Y. Li, Y. Song, X. Zhang, X. Wu, F. Wang and Z. Wang, Macromol. Chem. Phys., 2015, 216, 113–121 CrossRef CAS.
- A. S. Rewar, H. D. Chaudhari, R. Illathvalappil, K. Sreekumar and U. K. Kharul, J. Mater. Chem. A, 2014, 2, 14449–14458 RSC.
- Y. Ren, J. Guo, Q. Lu, D. Xu, J. Qin and F. Yan, ChemSusChem, 2018, 11, 1092–1098 CrossRef CAS PubMed.
- P. Y. Zheng, C. C. Ye, X. S. Wang, K. F. Chen, Q. F. An, K. R. Lee and C. J. Gao, J. Membr. Sci., 2016, 510, 220–228 CrossRef CAS.
- C. Yuan, J. Guo and F. Yan, Polymer, 2014, 55, 3431–3435 CrossRef CAS.
- Z. Zhang, Q. Zhao, J. Yuan, M. Antonietti and F. Huang, Chem. Commun., 2014, 50, 595–2597 Search PubMed.
- S. Zhou, N. Song, X. Lv and Q. Jia, J. Chromatogr. A, 2018, 1565, 19–28 CrossRef CAS PubMed.
- J. K. Sun, W. Zhang, R. Guterman, H. J. Lin and J. Yuan, Nat. Commun., 2018, 9, 1717 CrossRef PubMed.
- T. Kitao, Y. Zhang, S. Kitagawa, B. Wang and T. Uemura, Chem. Soc. Rev., 2017, 46, 3108–3133 RSC.
- J. Chen, M. Zhong, L. Tao, L. Liu, S. Jayakumar, C. Li, H. Li and Q. Yang, Green Chem., 2018, 20, 903–911 RSC.
- Q. Sun, B. Aguila, J. Perman, N. Nguyen and S. Ma, J. Am. Chem. Soc., 2016, 138, 15790–15796 CrossRef CAS PubMed.
- W. Wang, C. Li, L. Yan, Y. Wang, M. Jiang and Y. Ding, ACS Catal., 2016, 6, 6091–6100 CrossRef CAS.
- M. Ding and H.-L. Jiang, ACS Catal., 2018, 8, 3194–3201 CrossRef CAS.
- Q. Guo, C. Chen, L. Zhou, X. Lia, Z. Li, D. Yuan, J. Ding, H. Wan and G. Guan, Microporous Mesoporous Mater., 2018, 261, 79–87 CrossRef CAS.
- L. Hao, P. Li, T. Yang and T. S. Chung, J. Membr. Sci., 2013, 436, 221–231 CrossRef CAS.
- A. R. Nabais, A. P. S. Martins, V. D. Alves, J. G. Crespo, I. M. Marrucho, L. C. Tomé and L. A. Neves, Sep. Purif. Technol., 2019, 222, 168–176 CrossRef CAS.
- J. F. Olorunyomi, K. Y. Chan, L. Gao and C. Y. V. Li, Microporous Mesoporous Mater., 2018, 259, 255–263 CrossRef CAS.
- L. Gao, C. Ying, V. Li, K. Y. Chan and Z. N. Chen, J. Am. Chem. Soc., 2014, 136, 7209–7212 CrossRef CAS PubMed.
- M. Isik, T. Lonjaret, H. Sardon, R. Marcilla, T. Herve, G. G. Malliaras, E. Ismailova and D. Mecerreyes, J. Mater. Chem. C, 2015, 3, 8942–8948 RSC.
- M. Isik, R. Gracia, L. C. Kollnus, L. C. Tomé, I. M. Marrucho and D. Mecerreyes, ACS Macro Lett., 2013, 2, 1975–1979 Search PubMed.
- K. Grygiel, B. Wicklein, Q. Zhao, M. Eder, T. Pettersson, L. Bergström, M. Antoniettia and J. Yuan, Chem. Commun., 2014, 50, 12486–12489 RSC.
- B. Zhang, G. Sudre, G. Quintard, A. Serghei, L. David, J. Bernard, E. Fleury and A. Charlot, Carbohydr. Polym., 2017, 157, 586–595 CrossRef CAS PubMed.
- P. Zhang, J. Yuan, T. P. Fellinger, M. Antonietti, H. Li and Y. Wang, Angew. Chem., Int. Ed., 2013, 52, 6028–6032 CrossRef CAS PubMed.
- W. Zhang, H. Shimakoshi, N. Houfuku, X. M. Song and Y. Hisaeda, Dalton Trans., 2014, 43, 13972–13978 RSC.
- K. Nakashima, N. Kamiya, D. Koda, T. Maruyamac and M. Goto, Org. Biomol. Chem., 2009, 7, 2353–2358 RSC.
- A. Grollmisch, U. Kragl and J. Großeheilmann, SynOpen, 2018, 2, 192–199 CrossRef.
- K. Manojkumar, K. T. Prabhu Charan, A. Sivaramakrishna, P. C. Jha, V. M. Khedkar, R. Siva, G. Jayaraman and K. Vijayakrishna, Biomacromolecules, 2015, 16, 894–903 CrossRef CAS PubMed.
- J. L. Freyer, S. D. Brucks, G. S. Gobieski, S. T. Russell, C. E. Yozwiak, M. Sun, Z. Chen, Y. Jiang, J. S. Bandar, B. R. Stockwell, T. H. Lambert and L. M. Campos, Angew. Chem., Int. Ed., 2016, 55, 12382–12386 CrossRef CAS PubMed.
- H. Zhang, C. Liu, L. Zheng, W. Feng, Z. Zhou and J. Nie, Electrochim. Acta, 2015, 159, 93–101 CrossRef CAS.
- J. Yuan and M. Antonietti, Macromolecules, 2011, 44, 744–750 CrossRef CAS.
- J. Zamory, M. Bedu, S. Fantini, S. Passerini and E. Paillard, J. Power Sources, 2013, 240, 745–752 CrossRef.
- A. Vizintin, R. Guterman, J. Schmidt, M. Antonietti and R. Dominko, Chem. Mater., 2018, 30, 5444–5450 CrossRef CAS.
- L. Li, T. A. Pascal, J. G. Connell, F. Y. Fan, S. M. Meckler, L. Ma, Y. M. Chiang, D. Prendergast and B. A. Helms, Nat. Commun., 2017, 8, 2277 CrossRef PubMed.
- Y. S. Ye, J. Rick and B. J. Hwang, J. Mater. Chem. A, 2013, 1, 2719–2743 RSC.
- J. Le Bideau, L. Viau and A. Vioux, Chem. Soc. Rev., 2011, 40, 907–925 RSC.
- M. G. Cowan, A. M. Lopez, M. Masuda, Y. Kohno, W. M. McDanel, R. D. Noble and D. L. Gin, Macromol. Rapid Commun., 2016, 37, 1150–1154 CrossRef CAS PubMed.
- P. H. Wang, T. L. Wang, W. C. Lin, H. Y. Lin, M. H. Lee and C. H. Yang, Nanomaterials, 2018, 8, 225 CrossRef PubMed.
- P. S. C. de Oliveira, S. A. Alexandre, G. G. Silva, J. P. C. Trigueiro and R. L. Lavall, Eur. Polym. J., 2018, 108, 452–460 CrossRef CAS.
- M. G. Cowan, D. L. Gin and R. D. Noble, Acc. Chem. Res., 2016, 49, 724–732 CrossRef CAS PubMed.
- D. L. Gin and R. D. Noble, Science, 2011, 332, 674–676 CrossRef CAS PubMed.
- Z. Dai, R. D. Noble, D. L. Gin, X. Zhang and L. Deng, J. Membr. Sci., 2016, 497, 1–20 CrossRef CAS.
- L. C. Tomé and I. M. Marrucho, Chem. Soc. Rev., 2016, 45, 2785–2824 RSC.
- L. C. Tomé, D. Mecerreyes, C. S. R. Freire, L. P. N. Rebel and I. M. Marrucho, J. Membr. Sci., 2013, 428, 260–266 CrossRef.
- J. E. Bara, E. S. Hatakeyama, D. L. Gin and R. D. Noble, Polym. Adv. Technol., 2008, 19, 1415–1420 CrossRef CAS.
- P. Li, K. P. Pramoda and T. S. Chung, Ind. Eng. Chem. Res., 2011, 50, 9344–9353 CrossRef CAS.
- T. K. Carlisle, G. D. Nicodemus, D. L. Gin and R. D. Noble, J. Membr. Sci., 2012, 397–398, 24–37 CrossRef CAS.
- S. V. Shaligram, P. P. Wadgaonkar and U. K. Kharul, J. Membr. Sci., 2015, 493, 403–413 CrossRef CAS.
- A. S. L. Gouveia, L. Ventaja, L. C. Tomé and I. M. Marrucho, Membranes, 2018, 8, 124 CrossRef PubMed.
- M. Ambrogi, K. Täuber, M. Antonietti and J. Yuan, J. Mater. Chem. A, 2015, 3, 5778–5782 RSC.
- R. O. Mäkiniemi, P. Das, D. Hönders, K. Grygiel, D. Cordella, C. Detrembleur, J. Yuan and A. Walther, ACS Appl. Mater. Interfaces, 2015, 7, 15681–15685 CrossRef PubMed.
- G. Hernández, M. Işik, D. Mantione, A. Pendashteh, P. Navalpotro, D. Shanmukaraj, R. Marcilla and D. Mecerreyes, J. Mater. Chem. A, 2017, 5, 16231–16240 RSC.
- D. Wu, Y. Yu, J. Zhang, L. Guo and Y. Kong, ACS Appl. Mater. Interfaces, 2018, 10, 23362–23368 CrossRef CAS PubMed.
- N. Pothanagandhi, A. Sivaramakrishna and K. Vijayakrishna, Polym. Chem., 2017, 8, 918–925 RSC.
Footnotes |
| † Dedicated to Professor Markus Antonietti on the occasion of his 60th birthday. |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |