Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies
Pallab
Barai
a,
Kenneth
Higa
b and
Venkat
Srinivasan
*a
aArgonne National Laboratory, Lemont, Illinois 60439, USA. E-mail: vsrinivasan@anl.gov
bLawrence Berkeley National Laboratory, Berkeley, California 94720, USA
First published on 15th January 2020
Abstract
Correction for ‘Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies’ by Pallab Barai et al., Phys. Chem. Chem. Phys., 2017, 19, 20493–20505.
The authors would like to point out an error in one of the equations. The overpotential (η) used in eqn (2) of the main manuscript has been defined as η = ϕs − ϕe − ULi (see left column of the fourth page). Instead the overpotential term should be, η = ϕs − ϕe − ULi + (Δμe−/F).1,2 Here, ϕs is the solid phase potential, ϕe is the electrolyte potential, ULi is the open circuit potential of lithium metal, F indicates the Faraday constant, and Δμe− is the change in electrochemical potential due to the mechanical stress induced effects. The form of eqn (2) shown in the published version of the manuscript takes into account the impact of mechanical stress induced change in electrochemical potential (Δμe−) in the pre-exponential factor, but the effect of Δμe− was erroneously neglected in the overpotential (η) term, which appears inside the exponential.
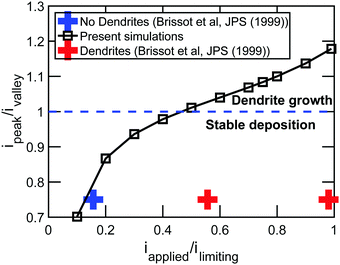
The correction of the overpotential term does not change the qualitative results shown in the paper; however, it allows better quantitative predictions. To demonstrate how this correction in the overpotential term of the Butler–Volmer equation affects some of the results, we recreate the two key figures in the manuscript, namely, Fig. 4 and 6, with the updated form of overpotential (η). The modified figures are shown below. It is evident from the corrected figures that the relative magnitude of current density at the peak and the valley change due to incorporation of the extra term into the overpotential expression. However, the overall trend did not change significantly. Such as, enhanced dendrite growth at higher current densities is still evident from the corrected version of Fig. 4. Similarly, it can still be concluded from the modified Fig. 6 that high modulus electrolytes demonstrate improved suppression of the dendritic protrusion.
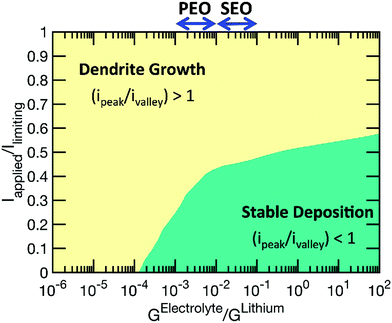 | ||
| Fig. 6 (Corrected) A phase map of the applied current with respect to the shear modulus of the polymer electrolyte phase. This map indicates that by increasing the elastic modulus of the electrolyte it may be possible to operate the battery at higher currents without the formation of dendrites. Elastic properties of poly(ethylene oxide) (PEO) and poly(styrene ethylene oxide) (SEO) have been adopted from Mullin et al.3 | ||
We suggest that researchers use the corrected equations in further studies.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- C. Monroe and J. Newman, J. Electrochem. Soc., 2004, 151, A880–A886 CrossRef CAS.
- C. Monroe and J. Newman, J. Electrochem. Soc., 2005, 152, A396–A404 CrossRef CAS.
- S. Mullin, A. Panday, N. P. Balsara, M. Singh, H. B. Eitouni and E. D. Gomez, US Pat., US 2009/0104523 A1, 2009 Search PubMed.
| This journal is © the Owner Societies 2020 |