How external perturbations affect the chemoselectivity of substrate activation by cytochrome P450 OleTJE†
Abstract
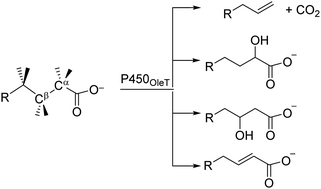
Cytochrome P450 enzymes are versatile biocatalysts found in most forms of life. Generally, the cytochrome P450s react with dioxygen and hence are haem-based mono-oxygenases; however, in specific isozymes, H2O2 rather than O2 is used and these P450s act as peroxygenases. The P450 OleTJE is a peroxygenase that binds long to medium chain fatty acids and converts them to a range of products originating from Cα-hydroxylation, Cβ-hydroxylation, Cα–Cβ desaturation and decarboxylation of the substrate. There is still controversy regarding the details of the reaction mechanism of P450 OleTJE; how the products are formed and whether the product distributions can be influenced by external perturbations. To gain further insights into the structure and reactivity of P450 OleTJE, we set up a range of large active site model complexes as well as full enzymatic structures and did a combination of density functional theory studies and quantum mechanics/molecular mechanics calculations. In particular, the work focused on the mechanisms leading to these products under various reaction conditions. Thus, for a small cluster model, we find a highly selective Cα-hydroxylation pathway that is preferred over Cβ–H hydrogen atom abstraction by at least 10 kcal mol−1. Introduction of polar residues to the model, such as an active site protonated histidine residue or through external electric field effects, lowers the Cβ–H hydrogen atom abstraction barriers are lowered, while a full QM/MM model brings the Cα–H and Cβ–H hydrogen atom abstraction barriers within 1 kcal mol−1. Our studies; therefore, implicate that environmental effects in the second-coordination sphere can direct and guide selectivities in enzymatic reaction mechanisms.
- This article is part of the themed collection: Quantum Theory: The Challenge of Transition Metal Complexes


 Please wait while we load your content...
Please wait while we load your content...