Entangled iodine and hydrogen peroxide formation in ice†
Abstract
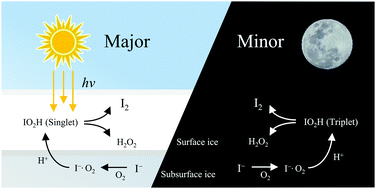
Ice-core records show that anthropogenic pollution has increased the global atmospheric concentrations of hydrogen peroxide and iodine since the mid-20th century. Here, for the first time, we demonstrate a highly efficient mechanism that synergistically produces them in icy water conditions. This reaction is aided by a key intermediate IO2H, formed by an I− ion with a dissolved O2 in acidic icy water, which produces both I as well as O2H radicals. I recombines with I− to produce I2− at a diffusion-limited rate, followed by formation of I3− through disproportionation, while O2H yields H2O2 with I− and a proton dissolved in icy water.
- This article is part of the themed collection: Environmental Science: Atmospheres – Introducing our new Advisory Board Members


 Please wait while we load your content...
Please wait while we load your content...