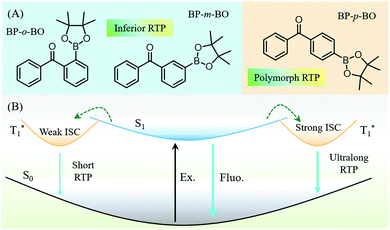
Elucidation of distinct fluorescence and room-temperature phosphorescence of organic polymorphs from benzophenone–borate derivatives†
Weilong
Che
a,
Yanbin
Gong
b,
Liangjing
Tu
a,
Mengmeng
Han
b,
Xiaoning
Li
a,
Yujun
Xie
 *a and
Zhen
Li
*abc
*a and
Zhen
Li
*abc
aInstitute of Molecular Aggregation Science, Tianjin University, Tianjin, 300110, P. R. China. E-mail: xieyujun@tju.edu.cn
bDepartment of Chemistry, Wuhan University, Wuhan, 430000, P. R. China
cJoint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Binhai New City, Fuzhou, 350207, P. R. China
First published on 28th August 2020
Abstract
The development of organic room-temperature phosphorescence (RTP) is accompanied by opportunities and challenges. RTP from crystal polymorphism has aroused much attention, due to the significant different photophysical characteristics and intermolecular packings found in the same molecule with different crystal phases. Herein, we report three organic molecules BP-o-BO, BP-m-BO, and BP-p-BO, in which two crystal polymorphisms of BP-p-BO are successfully cultivated with different emission properties. BP-p-BO-A exhibits bright cyan photoluminescence (PL) with a quantum yield of 11.3% and a distinct RTP with a lifetime of 17.1 ms, which is much higher than the deep blue PL of BP-p-BO-B (6.9%) and the corresponding RTP lifetime of 3.3 ms. Crystal structure analyses indicate that the different emission properties can be ascribed to the different intermolecular packing, further demonstrating the essential role of molecular packing in the designing of RTP materials.
Introduction
Room-temperature phosphorescence (RTP) is a novel optical phenomenon, in which the luminescence can last for seconds, minutes, and even hours after the removal of the excitation light source.1–4 At present, RTP materials have attracted increasing attention due to their characteristic of a long lifetime for practical applications, such as displays, emergency signs, information storage, encryption, bioimaging, etc.5–8 However, most of the reported RTP luminophors are restricted to inorganic noble-metal materials, owing to the efficient intersystem crossing (ISC) from singlet to the triplet states enhanced by the large spin–orbit coupling (SOC) effect.2,9 By contrast, pure organic materials exhibit an advantageous alternative to their inorganic counterpart, including the low-cost, excellent biocompatibility, adjustable photoelectric properties, etc.10–16 However, the RTP phenomenon is relatively hard to be observed in organic compounds, because of the spin-forbidden transition between singlet and triplet states. Also, the triplet excitons are easily quenched by the intense intramolecular vibration and oxygen in the air. Excitingly, recent investigations revealed that the introduction of heavy halogen atoms, carbonyls groups, hydrogen bonding, H-aggregation, some heteroatoms, etc. are supposed to facilitate the ISC process.17–27 Accompanied by the rigid molecular environment in the crystal, host–guest cocrystals, or the polymer matrix, RTP can be intensified because of the restricted molecular vibration and the isolated oxygen environment.28–31 To date, much attention has been focused on how intermolecular packing arrangement affects the RTP character in the crystal state,32–34 due to the crucial role of molecular packing in regulating the luminous efficiency, emission wavelength,35,36 and lifetime.37,38 However, despite the significant advances, the design of highly efficient RTP compounds is still a great challenge, because of the complicated impact factors and ambiguous inherit mechanism.21Due to the relatively explicit relationship between the molecular configuration and RTP performance, it is still an effective method to realize rich and colorful RTP through regulating molecular packing in the crystal state.39–41 However, organic polymorphism provides a perfect opportunity to evaluate RTP properties from the perspective of intermolecular packing, because RTP is derived from the same molecule embedded in different crystalline phases.42,43 Meanwhile, the polymorphism presents the diverse RTP emission, and the visualization of different intermolecular interactions helps in optimizing the molecular design strategy and achieve good photophysical performance,44–48 which have aroused broad interest.49–51 For example, Wang et al. disclosed the different emission colors in four organic polymorphs with different molecular arrangements.52 In our recent work, three polymorphs of phenothiazine derivatives (CzS-CN) with different RTPs and aggregation-induced emission (AIE) properties were successfully cultivated, and another two polymorphs of ortho-substituted triphenylamine derivatives exhibited RTP and mechanoluminescence (ML) properties,53 respectively. These findings have undoubtedly revealed the different photophysical features in polymorphs, greatly promoting the understanding of how molecular packing affects the luminous properties.54,55 However, the organic RTP is derived from the radiative transition of triplet states, which is strongly determined by the molecular configuration and intermolecular packing. So far, the research on polymorphism is still relatively scarce, although the relevant work will greatly promote the exploration of the triplet excited dynamic process in different molecular conformations and packings.56
The benzophenone group has been reported to be very efficient in producing triplet excitons under photoirradiation, and their derivatives exhibit a highly phosphorescence quantum yield with various colors at room temperature, as reported by the Tang group.57 Actually, the substitution of hydrogen atoms in benzophenone would cause a significant modification in the molecular configuration and the excited state energy levels. Moreover, the high production efficiency of triplet excitons is beneficial to deliver excitation energy to traps, thus giving rise to the novel RTP in the solid state.58 Boronic esters are one of the promising candidates for designing RTP materials, and the boron atom can communicate very well in the form of p–π conjugation with the phenyl group because of the empty p-orbital.59,60 Accompanied by the oxygen atoms in borate, the ISC transition should be efficient due to the enhanced n–π* transition.61 The oxygen atoms can also strengthen the intermolecular hydrogen-bonding in the crystal state, thus prohibiting the non-radiative transition. In this work, we report three metal- and halogen-free isomers of BP-o-BO, BP-m-BO, and BP-p-BO, composed of benzophenone (BP) and dioxaborolane (BO) moieties (Fig. 1). These isomers exhibit distinct excited state characteristics due to the different substitution positions. Interestingly, a unique polymorph-dependent emission is found in BP-p-BO. The BP-p-BO-A crystal is cultivated by rapid crystallization in the mixture solution of petroleum ether/dichloromethane, while the slow crystallization yields polymorphic crystal BP-p-BO-B with a significantly different crystal structure. It is impressive that the green RTP of BP-p-BO-A can be observed after ceasing the UV light irradiation, which cannot be observed in the counterpart. The PL quantum yields (PLQY) of BP-p-BO-A and BP-p-BO-B are 11.3% and 6.9% respectively, with lifetimes of 17.1 ms and 3.3 ms. By contrast, BP-o-BO and BP-m-BO exhibit inferior luminous efficiencies of 0.5% and 0.6% with RTP lifetimes of 63 ms and 53 ms, respectively.
Results and discussion
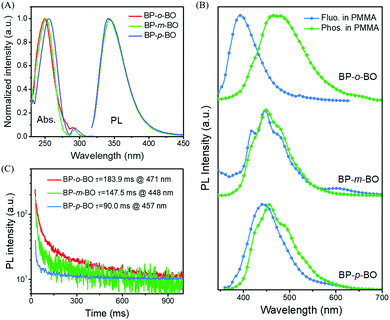
The synthetic routes of three isomers are presented in Scheme S1 of the ESI.† The one-step reaction gave high yields around 70%, and the products were fully characterized by 1H, 13C NMR, high resolution mass spectroscopy (Fig. S1–S9, ESI†), and elemental analysis. BP-o-BO, BP-m-BO, and BP-p-BO have good thermal stability with relatively high Td95% temperatures of 201, 214, and 223 °C (Fig. S10A, ESI†), while the corresponding melting points are 115.7, 99.7, and 121.7 °C, respectively, according to the DSC curves (Fig. S10B, ESI†). It should be noted that the melting points of BP-p-BO-A and BP-p-BO-B are identical regardless of the different crystal forms.The photophysical properties of the isomers are investigated in both solution and crystal state through UV-visible absorption, steady-state photoluminescence (PL), and time-resolved PL spectroscopy. The isomers show good solubility in common organic solvents. In dilute tetrahydrofuran (THF) solution (5.0 × 10−5 M), BP-o-BO, BP-m-BO and BP-p-BO exhibit similar absorption spectra, which are composed of the strong π → π* absorption band around 250 nm and the weak n → π* absorption band around 290 nm (Fig. 2A), respectively. Relatively, the absorption spectrum of BP-p-BO slightly red-shifts compared to that of counterparts, indicating the better conjugation degree of para substitution. The steady-state PL spectra of isomers are identical in THF solution with a maximum emission peak at 341 nm (Fig. 2A). Upon increasing the water fraction in the water/THF solution, the fluorescence intensity gradually decreases to abscissa accompanied by a red-shifted emission (Fig. S11–S13, ESI†). When the temperature is cooled to 77 K, the nonradiative decay of the triplet state is largely suppressed in rigid freezing circumstance. In glassy THF solution (5.0 × 10−5 M), the isolated BP-o-BO and BP-m-BO present similar fluorescence and phosphorescence profiles peaked at 393, 415, 445 and 478 nm, indicating the similar singlet and triplet excited state energy levels originated from the same chromophore of BP (Fig. S14A and C, ESI†). In contrast, the corresponding spectra of BP-p-BO slightly red-shifted, with emission peaks at 427, 454, 488 and 529 nm, respectively (Fig. S14E, ESI†). Besides, it is surprising to find that BP-o-BO, BP-m-BO, and BP-p-BO have ultralong phosphorescence lifetimes of 2.22 (416 nm), 2.26 (415 nm), and 1.68 s (427 nm), respectively (Fig. S14B, D and F, ESI†), implying the high phosphorescence efficiency of benzophenone at cryogenic temperature. The considerable overlap of PL and phosphorescence spectra at 77 K also revealed the efficient ISC process between the singlet and triplet states. The photographs in Fig. S15 (ESI†) present the deep-blue ultralong phosphorescence recorded at 77 K after ceasing 365 nm UV irradiation.
The PL properties in the polymethyl methacrylate (PMMA) matrix are also investigated. The samples and PMMA are dissolved in THF with a weight ratio of 3%, and the transparent uniform films can be obtained after the solution is dried over in glass tubes. Due to the strong ISC effect of the BP group, the PMMA doped films show detectable RTP, however, it is not visible to naked eyes because of the weak intermolecular interactions. The fluorescence and phosphorescence spectra are very different compared to those in THF solution. BP-o-BO, BP-m-BO, and BP-p-BO demonstrate the intense fluorescence peaked at 393, 446, and 443 nm, accompanied by the weak phosphorescence centered at 471, 448, and 457 nm (Fig. 2B) with lifetimes of 183.9, 147.5, and 90.0 ms, respectively (Fig. 2C). However, once the PMMA matrix is cooled to 77 K, their photophysical properties are almost identical, due to the similar fine emission bands of PL and phosphorescence spectra (Fig. S16, ESI†). The phosphorescence lifetimes are different at different emission bands, ranging in 5.2–40.5 ms for BP-o-BO and 6.1–13.2 ms for BP-p-BO (Fig. S16C, ESI†). It is surprising to find the intense phosphorescence of BP-m-BO with an ultralong lifetime of about 2.02–2.33 s (Fig. S16F, ESI†). As shown in Fig. S17 of the ESI,† all the isomers show sky-blue emissions under 365 nm UV light, and the emissions turn to cyan and lasts for several seconds upon ceasing UV irradiation.
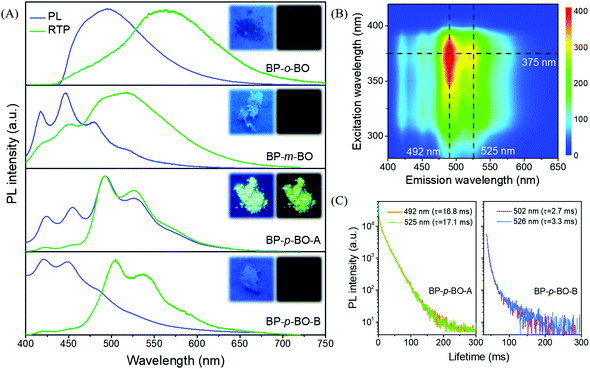
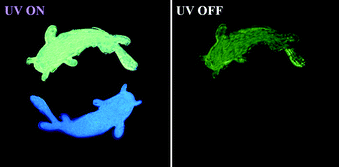
In sharp contrast to that in the PMMA matrix, the isomers exhibit much stronger emission in the crystalline state. To elucidate the correlation between RTP characteristics and molecular packing, single crystals are successfully cultivated by the solvent evaporation from the mixture solution of petroleum ether and dichloromethane. Excitedly, two crystal polymorphs of BP-p-BO are obtained. The rapid crystallization produces the BP-p-BO-A crystal, while the slow crystallization produces the BP-p-BO-B crystal. The polymorph BP-p-BO-A is not easy to cultivate, which requires a precise control of the evaporation speed. It is interesting to find that the polymorphs display different photophysical properties. As shown in Fig. 3A, a considerable spectral overlap can be observed between the PL and RTP spectra in three compounds, indicating the efficient ISC transition between singlet and triplet states in the crystal state. The BP-o-BO crystal exhibits blue-green fluorescence peaked at 494 nm and a weak RTP band around 566 nm. BP-m-BO shows prominent fluorescence emission bands in the range of 417–480 nm, but only a faint RTP band at 519 nm. The RTP lifetimes of BP-o-BO and BP-m-BO are 63 and 53 ms, respectively (Fig. S18A and B, ESI†). However, their RTP is too weak to be seen by the naked eye (Fig. 3A, insets). In BP-p-BO, although the polymorphs exhibit similar PL bands, the maximum emission of BP-p-BO-A is peaked at 421 and 450 nm, which is red-shifted to 492 and 525 nm in BP-p-BO-B accompanied by the inferior emission intensity. The insets display the emission photographs of polymorphs under and removal of 365 nm UV light irradiation. The emission color of BP-p-BO-A changes from cyan to green, while that of BP-p-BO-B is blue under UV light but turns to lightless when the irradiation is ceased (Fig. 3A). Fig. 3B presents the excitation-RTP mapping of BP-p-BO-A. The excitation wavelength is in the range of 310 to 390 nm, and the strongest RTP is excited by 375 nm. The PL quantum yield measurement is further carried out, but the pure fluorescence and phosphorescence heavily overlap in all emission bands, making them difficult to be fully discriminated, and thus, only a gross PL quantum yield can be obtained. BP-p-BO-A exhibits bright PL with a quantum yield up to 11.3%, which is higher than that of BP-p-BO-B (6.9%). Besides, BP-p-BO-A displays two RTP bands peaked at 492 and 525 nm with lifetimes of 16.8 and 17.1 ms, while very weak RTP bands peaked at 502 and 526 nm are observed in BP-p-BO-B with short lifetimes of 2.7 and 3.3 ms, respectively (Fig. 3C). The higher PL efficiency of the isomers in crystalline than in the PMMA matrix has undoubtedly revealed the crucial effect of intermolecular interactions on luminous properties.
At cryogenic temperature of 77 K, the nonradiative decay of the singlet and triplet excited states is largely suppressed because of the largely inhibited molecular vibration, therefore, brighter phosphorescence with a longer lifetime is observed. From Fig. S19A (ESI†), the strong PL and phosphorescence exhibit a similar fine vibrational spectra structure, resulting in visible phosphorescence. The phosphorescence of BP-p-BO-A and -B can last for more than 2 seconds with a lifetime of 0.25 s after the removal of the excitation source (Fig. S20, ESI†). The PL spectra of BP-o-BO and BP-m-BO (at 77 K) also show significant enhancement in the crystalline state. However, BP-o-BO exhibits a shorter phosphorescence lifetime of 0.23 s (Fig. S21, ESI†). Surprisingly, the phosphorescence lifetime of BP-m-BO dramatically extends to 3.28 s (Fig. S21F, ESI†), when the crystal is emerged in liquid nitrogen, and the naked eye visible phosphorescence can last for about 20 s (Fig. S22, ESI†).
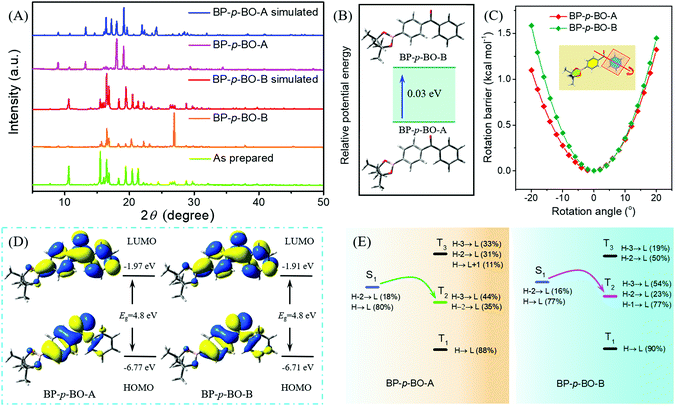
To gain a deeper understanding of the different photophysical properties of these compounds, the single-crystal structures are carefully analyzed with an emphasis on the intermolecular interactions and stacking patterns. The single crystals of BP-p-BO-A, BP-p-BO-B, BP-o-BO, and BP-m-BO belong to the P21/n, P21/c, P21/c, and P21/n space group, and the details of the cell parameters are listed in Table S1 (ESI†). The simulated X-ray diffraction (XRD) patterns of BP-p-BO-A and BP-p-BO-B correspond well with the measured one, and the different diffraction signals demonstrate the different polymorphs (Fig. 4A). In particular, some diffraction signals of the BP-p-BO-A crystals are even not found in the as-prepared sample, indicating the specificity character under the fast crystallization process. For better analyses, the phenyl group linked with the borate group is referred to as plane α, while another is referred to as plane β (Fig. S23, ESI†). The dihedral angles of plane α and β in BP-p-BO-A and BP-p-BO-B monomers are measured to be 53.0 and 57.4°, respectively. The smaller dihedral angle of BP-p-BO-A indicates a better conjugation degree. Despite the same molecules, BP-p-BO-A and BP-p-BO-B display intense and inferior RTP emission, respectively, and the different luminous properties can be ascribed to the different molecular packing. Four dimers are extracted from BP-p-BO-A and BP-p-BO-B crystals to display the explicit intermolecular interactions (Fig. 5A and B). Lots of hydrogen-bonding, including C–H⋯O (2.756–3.472 Å) and C–H⋯π (2.827–3.007 Å) are found in BP-p-BO-A, which contribute to reduce the energy annihilation, thereby increasing the RTP intensity and lifetime. By contrast, only C–H⋯O (2.441–3.481 Å) but no interactions between aromatic rings are observed in BP-p-BO-B (Fig. 5B). The UV absorption of crystals is presented in Fig. S24 (ESI†), from which it can be observed that BP-p-BO-A exhibits redder and stronger absorption around 380–400 nm than that of BP-p-BO-B, also indicating the stronger intermolecular coupling in BP-p-BO-A. It can be inferred that the inferior RTP emission of BP-p-BO-B can be ascribed to the weak coupling between aromatic groups. The formation of effective packing between chromophores is conducive to realize RTP emission.
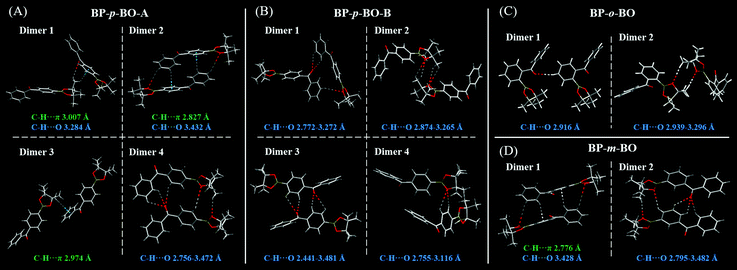 | ||
| Fig. 5 Crystal packing and intermolecular interactions of BP-p-BO-A (A), BP-p-BO-B (B), BP-o-BO (C), and BP-m-BO (D). | ||
The XRD patterns of BP-o-BO and BP-m-BO in crystal and as-prepared states are presented in Fig. S25 (ESI†), with clear diffraction signals corresponding well with the simulated patterns. In the monomer, a relatively larger dihedral angle of 66.1° between plane α and β is observed in BP-o-BO, while that of BP-m-BO is 51.0°. Two dimers are extracted from BP-o-BO (Fig. 5C) and BP-m-BO (Fig. 5D) crystals to reveal the intermolecular packing. A weak hydrogen-bonding of C–H⋯O (2.916–3.296 Å) is found in BP-o-BO, which should be the reason for the inferior RTP. BP-m-BO exhibits an almost identical but relatively looser intermolecular packing and interactions than that of the BP-p-BO-A (Fig. 5D), including C–H⋯O (2.795–3.482 Å) and C–H⋯π (2.776 Å). However, the very weak RTP emission of BP-m-BO indicates that not only the molecular packing, but also molecular configurations and the related molecular orbital energy should be considered for the RTP emission.
To explore the origin of relatively strong RTP and the prolonged lifetime of BP-p-BO-A, density functional theory (DFT) and time-dependent DFT (TDDFT) calculations are employed to unveil the inherent mechanism. On the consideration of the different crystallization processes of BP-p-BO-A and BP-p-BO-B, molecular potential energies and the rotational energy barrier of phenyl rings in the crystal are calculated by the ONIOM method. As shown in Fig. S26C and D (ESI†), molecular aggregates (composed of 100 molecules in BP-p-BO-A and 105 molecules in BP-p-BO-B) are extracted from the crystal structure, and divided into quantum mechanical (QM) and molecular mechanical (MM) regions, whereas the QM molecule in the high layer is described by the B3LYP/6-31G(d) method, while the MM molecules in the low layer are described by universal force field (UFF) with the incorporation of electronic embedding to provide a better description of the electrostatic interaction between the QM and MM regions. The rotational barrier energy of QM molecules is calculated through the energy of rotated geometry minus the energy at the equilibrium configuration, while the rotation angle is evaluated by the rotated dihedral angles relative to the equilibrium configuration. These results indicate that the BP-p-BO-A monomer is more stable and the potential energy is about 0.03 eV lower than that of BP-p-BO-B (Fig. 4B). However, when the phenyl ring (plane β) rotates through the C–C bond, the rotation barrier energy of BP-p-BO-B is higher than that of BP-p-BO-A (Fig. 4C). Therefore, it can be deduced that BP-p-BO-A has a better conjugation degree for the more stable molecular potential energy, while the molecular packing is more rigid in BP-p-BO-B, which can be verified by the higher crystal density of BP-p-BO-B than BP-p-BO-A (1.236 versus 1.232 g cm−3), and the followed molecular energy level analyses.
The photophysical properties of molecular aggregates are simulated using the identical theoretical model, and the singlet and triplet excited state energies of QM molecules are calculated by the TD-DFT method at the B3LYP/6-31G(d) level under the optimized S1 geometry to evaluate the ISC process. Because of the same molecular configuration, BP-p-BO-A and BP-p-BO-B reveal a similar frontier molecular orbital distribution. Their highest occupied molecular orbital (HOMO) spreads on the plane α and carbonyl group, while the lowest unoccupied molecular orbital (LUMO) distributes all over the molecule. The transition energies of singlet and the triplet excited states are presented in Fig. 4D, with the detail molecular orbital configurations listed in Table S2 of the ESI.† The calculated S1 state energy of BP-p-BO-A (2.85 eV) is relatively lower than that of BP-p-BO-B (2.90 eV), confirming the better conjugation degree and more stable molecular conformation. It has been reported that the singlet–triplet intersystem crossing would be efficient upon the close energy level gap (<0.3 eV), and the efficient transition route should be S1 → T2 in both BP-p-BO-A and BP-p-BO-B (Fig. 4E). Furthermore, the more similar transition configuration and isosurfaces in BP-p-BO-A indicate the stronger spin-orbital coupling probability and ISC process (Table S2, ESI†). The calculated results also prove the stronger transition between singlet and triplet states of BP-p-BO-A than that of BP-p-BO-B. The excited state properties of two dimers from BP-p-BO-A and BP-p-BO-B are analyzed because of the closer intermolecular interactions, and the results are listed in Fig. S27 and Table S3 (ESI†). It can be found that the excited singlet and triplet energies are lower than those in the monomer state, implying the conjugation effect in the dimers. The ISC channels of S1 → T2 are also found in the dimers, also indicating a stronger transition of BP-p-BO-A than BP-p-BO-B for the more transition routes. Under the optimized T1 geometry, the excited state transition configurations (Table S4, ESI†) exhibit similar characteristics to that of the S1 state. The dihedral angles between plane α and β of BP-p-BO-A and BP-p-BO-B in the optimized S1 and T1 geometry are also similar (Table S5, ESI†), indicating the similar spatial configuration at S1 and T1 states, and the detailed molecular geometry of BP-p-BO-A and BP-p-BO-B in crystal, S0, S1, and T1 states is listed in Tables S6 and S7, respectively.
The molecular aggregate properties of BP-o-BO (75 molecules, Fig. S26A, ESI†) and BP-m-BO (135 molecules Fig. S26B, ESI†) are also simulated by the ONIOM method. As for BP-o-BO, the HOMO spreads on the plane β and carbonyl group, while the LUMO is mainly offered by plane α (Fig. S28A, ESI†). Thus, the slightly separated HOMO and LUMO can be ascribed to the relatively large dihedral angles of plane α and β. However, the ISC transition route is inferior because of the relatively large energy gap (0.46 eV) under the excited states (S1 → T1) with the same orbital configuration (Fig. S28C, ESI†). In BP-m-BO, both the HOMO and LUMO distribute on the BP moiety (Fig. S28B, ESI†). Also owing to the same orbital configuration, two possible transition routes of S1 → T2 and S1 → T3 are found (Fig. S28D, ESI†), but the energy gap (0.22 and 0.3 eV) is larger than that in BP-p-BO-A, which should be the reason for the inferior RTP of BP-m-BO. The molecular geometry of BP-o-BO and BP-m-BO in crystal, S0, S1, and T1 states is also listed in Tables S8 and S9 (ESI†). Therefore, the calculation results correspond well with the RTP properties of four crystals.
Lastly, considering the different photophysical properties of the BP-p-BO polymorphs, the application presentation in the anti-counterfeiting is explored. As shown in Fig. 6, a simple encryption pattern is fabricated combined with the prominent RTP feature under the UV excitation. By controlling the crystalline growth rate, the micro-crystals of BP-p-BO-A and BP-p-BO-B are stamped on a parchment paper in a double goldfish shape. Under 365 nm UV light irradiation, the upper goldfish emits bright cyan light while the bottom goldfish emits deep blue light. After ceasing the UV irradiation, the visible green RTP of BP-p-BO-A can be readily visualized and lasts for seconds because of the long RTP lifetime. At the same time, the bottom goldfish of BP-p-BO-B is invisible due to the inferior RTP emission. Thus, the feasible application of the BP-p-BO in information encryption and decryption is successfully demonstrated, exhibiting the practical application of the polymorphs with different RTP intensities.
Conclusions
In summary, a series of pure organic luminogens based on benzophenone and borate with different PL properties are synthesized and investigated. Their fluorescence is weak in solutions, while a dual emission of fluorescence-RTP is observed in the crystal state. Interestingly, BP-p-BO exhibits unique polymorph-dependent emission characteristics, and BP-p-BO-A shows an obvious RTP with a long lifetime (17.1 ms) and a high PLQY (11.25%) compared to that of BP-p-BO-B (3.3 ms and 6.85%). The different RTP properties can be ascribed to the different molecular packing and the excited energies, and the effective close stacking in BP-p-BO-A contributes to the RTP emission. However, no visible RTP is observed in the isomers of BP-o-BO and BP-m-BO. The interesting RTP properties of BP-p-BO polymorphs indicate promising applications in anti-counterfeiting. The research results also prove the efficient phosphorescence efficiency of benzophenone derivatives and novel RTP molecular design strategies.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21905198), and acknowledge the starting Grants of Tianjin University, Tianjin Government, for financial support.Notes and references
- Y. Li, M. Gecevicius and J. R. Qiu, Chem. Soc. Rev., 2016, 45, 2090–2136 RSC.
- K. Van den Eeckhout, P. F. Smet and D. Poelman, Materials, 2010, 3, 2536–2566 CrossRef CAS.
- J. Yang, H. Gao, Y. Wang, Y. Yu, Y. Gong, M. Fang, D. Ding, W. Hu, B. Z. Tang and Z. Li, Mater. Chem. Front., 2019, 3, 1391–1397 RSC.
- L. Zhang, W.-L. Zhao, M. Li, H.-Y. Lu and C.-F. Chen, Acta Chim. Sin., 2020, 78 DOI:10.6023/A20060243.
- O. Kahn and C. J. Martinez, Science, 1998, 279, 44–48 CrossRef CAS.
- T. Maldiney, A. Bessiere, J. Seguin, E. Teston, S. K. Sharma, B. Viana, A. J. J. Bos, P. Dorenbos, M. Bessodes, D. Gourier, D. Scherman and C. Richard, Nat. Mater., 2014, 13, 418–426 CrossRef CAS.
- J. Yang, Z. Chi, W. Zhu, B. Z. Tang and Z. Li, Sci. China: Chem., 2019, 62, 1090–1098 CrossRef CAS.
- J. Yang and Z. Li, Chin. J. Org. Chem., 2019, 39, 3304–3305 CrossRef.
- B. Minaev, G. Baryshnikov and H. Agren, Phys. Chem. Chem. Phys., 2014, 16, 1719–1758 RSC.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS.
- A. Kishimura, T. Yamashita, K. Yamaguchi and T. Aida, Nat. Mater., 2005, 4, 546–549 CrossRef CAS.
- G. Marriott, R. M. Clegg, D. J. Arndt-Jovin and T. M. Jovin, Biophys. J., 1991, 60, 1374–1387 CrossRef CAS.
- Y. Xie and Z. Li, Chem. – Asian J., 2019, 14, 2524–2541 CrossRef CAS.
- J. Yang, M. Fang and Z. Li, InfoMat, 2020, 2, 791–806 CrossRef.
- A. J. Qin, Sci. China: Chem., 2018, 61, 635–636 CrossRef CAS.
- D. Wang, X. Wang, C. Xu and X. Ma, Sci. China: Chem., 2019, 62, 430–433 CrossRef CAS.
- S. Hirata, K. Totani, J. X. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386–3397 CrossRef CAS.
- C. R. Wang, Y. Y. Gong, W. Z. Yuan and Y. M. Zhang, Chin. Chem. Lett., 2016, 27, 1184–1192 CrossRef CAS.
- S. Hirata, Adv. Opt. Mater., 2017, 5, 1700116 CrossRef.
- S. Mukherjee and P. Thilagar, Chem. Commun., 2015, 51, 10988–11003 RSC.
- S. Xu, R. F. Chen, C. Zheng and W. Huang, Adv. Mater., 2016, 28, 9920–9940 CrossRef CAS.
- M. Baroncini, G. Bergamini and P. Ceroni, Chem. Commun., 2017, 53, 2081–2093 RSC.
- K. Kanosue and S. Ando, ACS Macro Lett., 2016, 5, 1301–1305 CrossRef CAS.
- L. Xiao and H. B. Fu, Chem. – Eur. J., 2019, 25, 714–723 CrossRef CAS.
- G. He, W. T. Delgado, D. J. Schatz, C. Merten, A. Mohammadpour, L. Mayr, M. J. Ferguson, R. McDonald, A. Brown, K. Shankar and E. Rivard, Angew. Chem., Int. Ed., 2014, 53, 4587–4591 CrossRef CAS.
- D. Chaudhuri, E. Sigmund, A. Meyer, L. Rock, P. Klemm, S. Lautenschlager, A. Schmid, S. R. Yost, T. Van Voorhis, S. Bange, S. Hoger and J. M. Lupton, Angew. Chem., Int. Ed., 2013, 52, 13449–13452 CrossRef CAS.
- L. Zhang, M. Li, Q. Y. Gao and C. Chen, Chin. J. Org. Chem., 2020, 40, 516–520 CrossRef.
- Y. Y. Gong, G. Chen, Q. Peng, W. Z. Yuan, Y. J. Xie, S. H. Li, Y. M. Zhang and B. Z. Tang, Adv. Mater., 2015, 27, 6195–6201 CrossRef CAS.
- M.-M. Fang, J. Yang and Z. Li, Chin. J. Polym. Sci., 2019, 37, 383–393 CrossRef CAS.
- Y. Wang, J. Yang, M. Fang, Y. Yu, B. Zou, L. Wang, Y. Tian, J. Cheng, B. Z. Tang and Z. Li, Matter, 2020, 2, 449–463 CrossRef.
- Z. Yan, L. Zou and X. Ma, Chin. J. Org. Chem., 2020, 40, 1814–1822 CrossRef.
- Z. F. Chai, C. Wang, J. F. Wang, F. Liu, Y. J. Xie, Y. Z. Zhang, J. R. Li, Q. Q. Li and Z. Li, Chem. Sci., 2017, 8, 8336–8344 RSC.
- J. Yang, X. Zhen, B. Wang, X. Gao, Z. Ren, J. Wang, Y. Xie, J. Li, Q. Peng, K. Pu and Z. Li, Nat. Commun., 2018, 9, 840 CrossRef.
- M. Fang, J. Yang, X. Xiang, Y. Xie, Y. Dong, Q. Peng, Q. Li and Z. Li, Mater. Chem. Front., 2018, 2, 2124–2129 RSC.
- H. W. Wu, C. Hang, X. Li, L. Y. Yin, M. J. Zhu, J. Zhang, Y. Y. Zhou, H. Agren, Q. Zhang and L. L. Zhu, Chem. Commun., 2017, 53, 2661–2664 RSC.
- Q. Li and Z. Li, Acc. Chem. Res., 2020, 53, 962–973 CrossRef CAS.
- H. W. Wu, W. J. Chi, G. Baryshnikov, B. Wu, Y. F. Gong, D. X. Zheng, X. Li, Y. L. Zhao, X. G. Liu, H. Agren and L. L. Zhu, Angew. Chem., Int. Ed., 2019, 58, 4328–4333 CrossRef CAS.
- Y. Xie, Y. Ge, Q. Peng, C. Li, Q. Li and Z. Li, Adv. Mater., 2017, 29, 1606829 CrossRef.
- Y. Wang, J. Yang, Y. Tian, M. Fang, Q. Liao, L. Wang, W. Hu, B. Z. Tang and Z. Li, Chem. Sci., 2020, 11, 833–838 RSC.
- Y. Tian, Y. Gong, Q. Liao, Y. Wang, J. Ren, M. Fang, J. Yang and Z. Li, Cell Rep. Phys. Sci., 2020, 1, 100052 CrossRef.
- X. Fang and D. Yan, Sci. China: Chem., 2018, 61, 397–401 CrossRef CAS.
- J. W. Chung, Y. You, H. S. Huh, B. K. An, S. J. Yoon, S. H. Kim, S. W. Lee and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 8163–8172 CrossRef CAS.
- Q. Q. Li and Z. Li, Sci. China Mater., 2020, 63, 177–184 CrossRef.
- X. S. Meng, B. Gui, D. Q. Yuan, M. Zeller and C. Wang, Sci. Adv., 2016, 2, e1600480 CrossRef.
- Z. Q. Xie, B. Yang, F. Li, G. Cheng, L. L. Liu, G. D. Yang, H. Xu, L. Ye, M. Hanif, S. Y. Liu, D. G. Ma and Y. G. Ma, J. Am. Chem. Soc., 2005, 127, 14152–14153 CrossRef CAS.
- Z. L. Zhang, Y. Zhang, D. D. Yao, H. Bi, I. Javed, Y. Fan, H. Y. Zhang and Y. Wang, Cryst. Growth Des., 2009, 9, 5069–5076 CrossRef CAS.
- X. Liu, Y. Jia, H. Jiang, G. Gao and M. Xia, Acta Chim. Sin., 2019, 77, 1194–1202 CrossRef.
- Y. Wang, P. Liu, Y. Lu and Y.-F. Men, Chin. J. Polym. Sci., 2016, 34, 1014–1020 CrossRef CAS.
- M. Ichikawa, R. Hibino, M. Inoue, T. Haritani, S. Hotta, T. Koyama and Y. Taniguchi, Adv. Mater., 2003, 15, 213–217 CrossRef CAS.
- Y. Ganin, Y. Takabayashi, P. Jeglic, D. Arcon, A. Potocnik, P. J. Baker, Y. Ohishi, M. T. McDonald, M. D. Tzirakis, A. McLennan, G. R. Darling, M. Takata, M. J. Rosseinsky and K. Prassides, Nature, 2010, 466, 221–225 CrossRef.
- C. Wang, Y. Yu, Z. Chai, F. He, C. Wu, Y. Gong, M. Han, Q. Li and Z. Li, Mater. Chem. Front., 2019, 3, 32–38 RSC.
- K. Wang, H. Y. Zhang, S. Y. Chen, G. C. Yang, J. B. Zhang, W. J. Tian, Z. M. Su and Y. Wang, Adv. Mater., 2014, 26, 6168–6173 CrossRef CAS.
- J. Wang, Z. Chai, J. Wang, C. Wang, M. Han, Q. Liao, A. Huang, P. Lin, C. Li, Q. Li and Z. Li, Angew. Chem., Int. Ed., 2019, 58, 17297–17302 CrossRef CAS.
- J. Yang, Z. C. Ren, B. Chen, M. M. Fang, Z. J. Zhao, B. Tang, Q. Peng and Z. Li, J. Mater. Chem. C, 2017, 5, 9242–9246 RSC.
- Y. Xie and Z. Li, Mater. Chem. Front., 2020, 4, 317–331 RSC.
- D. P. Yan, H. J. Yang, Q. Y. Meng, H. Y. Lin and M. Wei, Adv. Funct. Mater., 2014, 24, 587–594 CrossRef CAS.
- W. Z. Yuan, X. Y. Shen, H. Zhao, J. W. Y. Lam, L. Tang, P. Lu, C. Wang, Y. Liu, Z. Wang, Q. Zheng, J. Z. Sun, Y. Ma and B. Z. Tang, J. Phys. Chem. C, 2010, 114, 6090–6099 CrossRef CAS.
- D. I. Zloba, L. M. Buravtseva, O. S. Pyshkin and M. A. Strzhemechny, Low Temp. Phys., 2013, 39, 1103–1110 CrossRef CAS.
- Y. Shoji, Y. Ikabata, Q. Wang, D. Nemoto, A. Sakamoto, N. Tanaka, J. Seino, H. Nakai and T. Fukushima, J. Am. Chem. Soc., 2017, 139, 2728–2733 CrossRef CAS.
- J. Yang, Z. Ren, Z. Xie, Y. Liu, C. Wang, Y. Xie, Q. Peng, B. Xu, W. Tian, F. Zhang, Z. Chi, Q. Li and Z. Li, Angew. Chem., Int. Ed., 2017, 56, 880–884 CrossRef CAS.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323–5351 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis, structural information of the compounds (NMR, HRMS, and elemental analysis), absorption and emission spectra, procedures for the DFT calculations and crystallographic data. CCDC 2005208–2005211. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cp02881a |
| This journal is © the Owner Societies 2020 |