Excimer-origin CPL vs. monomer-origin magnetic CPL in photo-excited chiral binaphthyl-ester-pyrenes: critical role of ester direction†
Abstract
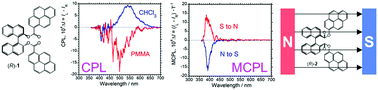
Two chiral binaphthyl (BNp) derivatives bearing oppositely oriented ester linkers to two pyrene (Py) moieties [(R)/(S)-1 and (R)/(S)-2] enabled Py-origin circularly polarized luminescence (CPL), magnetic CPL (MCPL), and circular dichroism (CD). (R)-1 that exhibited (−)-sign CD showed (+)-sign Py-excimer CPL but did not exhibit MCPL. Conversely, (R)-2, with (−)-sign CD, did not show excimer-origin CPL, but exhibited clear Py-monomer MCPL.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...