Visible-light responsive BiNbO4 nanosheet photoanodes for stable and efficient solar-driven water oxidation†
Abstract
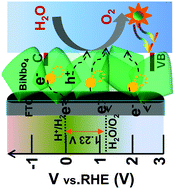
Herein, we report bismuth niobate (BiNbO4), which is regarded as an emerging photoanode material for sustainable photoelectrochemical (PEC) solar energy conversion. BiNbO4 possesses a direct bandgap (Eg) of ∼2.6 eV, and shows an appropriate band alignment for the water oxidation/reduction reaction. In this study, a simple sol–gel route followed by a spin coating method was applied to develop BiNbO4 nanosheets under the optimum annealing conditions. It is known that the annealing temperatures of 500 and 550 °C influence the crystallinity and PEC properties of BiNbO4 films. In particular, the 550 °C annealed film exhibited sharply improved crystalline properties, and rapidly enhanced PEC performance, which were accompanied by a photocurrent density of 0.45 mA cm−2 at 1.23 V vs. the reversible hydrogen electrode (RHE) (briefly abbreviated as 1.23 VRHE) in a strong alkaline solution of 1 M NaOH, compared with 0.26 mA cm−2 at 1.23 VRHE of the 500 °C annealed film. This may be attributed to the main increase of the crystallinity, as well as the improvement of the electronic properties. In addition, the BiNbO4 (550 °C) film showed an incident photon-to-current efficiency of 20% at 425 nm, and produced a stable photoresponse under light illumination in a strong alkaline solution over 5 h, compared with a BiVO4 electrode.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...