Adsorption geometry and self-assembling of chiral modifier (R)-(+)-1-(1-naphthylethylamine) on Pt(111)†
Abstract
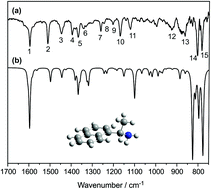
A mechanistic study on interaction of a chiral modifier – (R)-(+)-1-(1-naphthylethylamine) (R-NEA) – with a single crystalline Pt(111) surface is reported. The details of the adsorption geometry of individual R-NEA molecules and their intermolecular interactions are addressed by combination of infrared reflection absorption spectroscopy (IRAS) and scanning tunneling microscopy (STM). The spectroscopic observations suggest that the molecules are tilted with respect to the underlying metal substrate with the long axis of the naphthyl ring being parallel and the short axis tilted with respect to the surface. In the medium coverage range, formation of directed 3–5 membered chains was observed by STM for the first time, which points to intermolecular bonding between individual molecules and might account for an unusual tilted adsorption geometry deduced from the IR spectra. Based on the STM images revealing the atomic structure of the Pt grid close to the R-NEA chains, we propose the adsorption configuration of NEA fitting both the IRAS and STM data. The obtained results suggest that this strong intermolecular interaction energetically stabilizes the tilted adsorption geometry of the naphthyl ring, which otherwise would be expected to lie flat on the metal to maximize the dispersive interactions. At the coverage close to saturation, R-NEA builds a self-assembled overlayer with hexagonal symmetry, exhibiting intermolecular distances larger than in the directed chains.


 Please wait while we load your content...
Please wait while we load your content...