Sulfur hydrogen bonding and internal dynamics in the monohydrates of thenyl mercaptan and thenyl alcohol†
Abstract
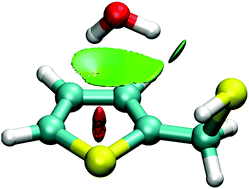
The monohydrates of thenyl alcohol and thenyl mercaptan have been probed in a supersonic jet expansion using chirped-pulse and Fabry–Perot Fourier-transform microwave spectroscopy. The rotational spectra revealed a single isomer for each of the dimers. The thenyl alcohol hydrate is stabilized by an O–H⋯Ow hydrogen bond between the alcohol and water, with water acting as a proton acceptor and additionally engaging in an Ow–H⋯π interaction with the thenyl ring. Conversely, water behaves as a proton donor in the thenyl mercaptan hydrate, linking to the thiol group though an Ow–H⋯S hydrogen bond and secondary Ow–H⋯π interactions with the ring. In both dimers water retains internal mobility, as tunneling doublings in the spectrum confirm an internal rotation motion of water inside the cluster. The experimental results have been complemented with density-functional-theory molecular orbital calculations, binding energy decomposition and a topological analysis of the electronic density, providing a comparative description of the effects of hydrogen bonding of water to the alcohol and thiol groups in the dimers, relevant to understand hydrogen bonding to sulfur centers.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...