Open Access Article
Open Access ArticleInduced magnetic field in sp-hybridized carbon rings: analysis of double aromaticity and antiaromaticity in cyclo[2N]carbon allotropes†
Nickolas D.
Charistos
 *a and
Alvaro
Muñoz-Castro
*a and
Alvaro
Muñoz-Castro
 *b
*b
aAristotle University of Thessaloniki, Department of Chemistry, Laboratory of Quantum and Computational Chemistry, Thessaloniki, 54 124, Greece. E-mail: nicharis@chem.auth.gr
bLaboratorio de Química Inorgánica y Materiales Moleculares, Facultad de Ingeniería, Universidad Autonoma de Chile, Llano Subercaceaux 2801, San Miguel, Santiago, Chile. E-mail: alvaro.munoz@uautonoma.cl
First published on 6th April 2020
Abstract
The induced magnetic field of C2N (N = 3–14) carbon rings was dissected to contributions from out-of-plane and in-plane π orbitals revealing two concurrent long range shielding or deshielding cones as a manifestation of the dual aromatic and antiaromatic character of C4n+2 and of C4n rings respectively. Aromaticity based on the magnetic criterion was evaluated with regard to the bonding pattern and geometrical characteristics that elucidate the influence of bond length and bond angle alteration on out-of-plane and in-plane magnetic responses. Ground state polyynic geometries of C4n+2 rings exhibit comparable shielding cones to annulenes, decreasing the magnetic response with regard to the ring size and similar πout and πin diatropicity. Transition state cumulenic rings display increased aromaticity expressed by a very strong constant magnetic response and augmented πout diatropicity with regard to πin. The variations of the induced magnetic field are explained on the basis of frontier orbital interactions through rotational excitations, which enable further rationalization of the aromatic/antiaromatic behavior.
Introduction
Since the discovery of buckminsterfullerene C60 in 1985,1 research on sp2 carbon allotropes such as fullerenes, graphenes2 and carbon nanotubes3 has sparked the interest on the design and synthesis of a plethora of novel carbon-based nanomaterials with exceptional diversity and applications in numerous fields such as optoelectronics, gas separation, energy conversion and shortage, catalysis and medicine.4–12 Around the same time research on different carbon allotropes with mixed sp2–sp hybridization13 or solely sp hybridized carbon atoms14 has paved the way for the discovery of new all carbon species with promising applications such as graphdiynes15–17 and carbon rings.18Early in 1989 Diederich et al. reported the gas phase generation of a highly reactive C18 cyclocarbon14 in which each carbon atom was bonded to two other carbons, in contrast to sp2 hybridized allotropes where the bonding pattern involves three-coordinated carbon atoms. The main question that evolved to a controversy by conflicting theoretical studies was on the bonding pattern of C18: is it polyynic with altering triple and single bonds, or cumulenic with equal bond lengths? Pure and hybrid DFT19–21 as well as MP222,23 calculations predict cumulenic structures, whereas HF,14,24 quantum Monte Carlo25 and CCSD26 calculations predict polyynic geometries. Further studies diagnosed that the DFT calculated bonding pattern depends on the amount of HF exchange of the hybrid functional,27,28 whereas range-separated exchange nonempirical DFT schemes provide polyynic structures regardless of the type of functional that is used.29 Finally in 2019 Kaiser et al.18 isolated by on-surface synthesis and structurally characterized C18 using atomic force microscopy (AFM), revealing a polyynic structure with altering single–triple CC bonds putting an end to the controversy.23,24 Moreover, the cumulenic structure has been predicted to be a transition state between two degenerate polyynic ground states with inverted single–triple bonds.25,28,30
A unique characteristic of sp-hybridized carbon structures is the presence of two perpendicular π-electron systems31 extending the capabilities from sp2-hybridized carbon structures which sustain a single π-system. Diederich proposed that C18 benefited from two distinctive sets of delocalized π electrons,14 one from out-of-plane (πout) and the other from in-plane (πin) oriented 18π orbitals (Fig. 1), both obeying Huckel's 4n + 2 rule, which is expected to exhibit interesting electron acceptor properties.27 The presence of double aromaticity for C4n+2 and antiaromaticity for C4n cyclocarbons was confirmed via ring current analysis by Fowler et al.,19 while the large NICS values reported by Suresh20 also imply the existence of double aromaticity given by the two perpendicular π-systems. The double aromaticity of small carbon rings and related boron–carbon molecules was also confirmed with the CMO-NICS analysis put forth by Schleyer et al.32 However, studies on the aromaticity of carbon rings were performed on cumulenic structures and the effect of bonding pattern on the magnetic response was not evaluated. Recently Baryshnikov et al.28 employed the GIMIC method to measure the ring currents of both polyynic and cumulenic configurations of C18 and reported that the polyynic structure induces a strong diatropic current which is however much weaker than the diatropic current of cumulene. Moreover, although the GIMIC method does not yet allow the dissection of ring current to contributions from different sets of orbitals, it obtained the current strengths of πout and πin sets by integrating the current density within distinctive contours and found that πout orbitals induce stronger diatropic currents than πin, both in polyynic and cumulenic geometries.
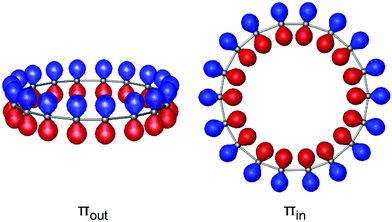 | ||
| Fig. 1 Illustration of two orthogonal sets of p orbitals of C18 ring displaying perpendicular to the plane (πout) and in-plane (πin) orientations. | ||
Herein, we study the distinctive characteristics of double aromaticity and antiaromaticity based on the magnetic criterion33 from cyclo[2N]carbon allotropes, given by their peculiar perpendicular π-systems. However we note that a thorough investigation of the aromaticity requires to take into consideration both electronic and energetic criteria.34–36 We perform a detailed analysis of the magnetic response of C2N (N = 3–14) rings which is directly related to the aromatic/antiaromatic behavior,37–42 by means of canonical orbital contributions to the induced magnetic field (CMO-IMF) that allows the through-space visualization of shielding and deshielding cones induced by different sets of orbitals, as well as from each CMO.43–46 Hence the magnetic field induced from each πout and πin set was visualized for polyynic and cumulenic geometries of C4n+2 rings, as well as for those of C4n rings, revealing the trends of the magnetic response of each set of MOs and the effect of ring size, bond length and bond angle. Our results show that polyynic geometries induce a cumulative magnetic field from both sets which is comparable to annulenes and declines with the evolution of ring size, whereas cumulenes induce a much stronger magnetic field independent of the ring size. Accordingly, C4n rings display decreasing deshielding cones for both sets with regard to the ring size. These trends are associated with the inherent bonding pattern and geometrical characteristics of carbon rings, which are further explained on the basis of frontier orbital interactions through rotational excitations.
Computational methods
A number of various exchange–correlation functionals (XCs) were tested for the optimization of C18 (see ESI,† Table S1). Functionals with a high percentage of HF exchange (HFX > 50%) yielded polyynic structures, whereas pure GGAs and functionals with a low percentage of HFX resulted in cumulenic structures with no bond length alteration (BLA) (Table S1, ESI†). In this work we have chosen the use of the range-separated hybrid ωB97XD47 for the optimization of the C2N (N = 3–14) molecules because it correctly reproduces the experimentally realized18 polyynic geometry of C18 and it has been reported to have the best performance among other functionals for the description of polyynic structures.48 Moreover, long-range corrected XC functionals like ωB97XD have been found to provide a better description of the cyclic electron delocalization in monocyclic and polycyclic aromatics with regard to conventional XC functionals.36 All geometry optimizations were carried out with Gaussian0949 using the 6-311++G(d,p) Gaussian basis set.Nucleus independent chemical shieldings (NICS) were calculated within the GIAO formalism using several functionals, as detailed in ESI,† and the triple-ζ slater type basis set with two polarization functions (TZ2P) employing the ADF2019 software.50,51 BHandHLYP52 was chosen for the quantitative analysis of dissected NICSzz because it performs in very good agreement with ωB97XD (Table S2 and Fig. S2, ESI†) and it is available for the CMO analysis of the chemical shift in ADF. The computationally affordable PBE53 was chosen for the qualitative analysis of the induced magnetic field. Although the PBE was found to overestimate the NICS, especially in polyynic structures, it accurately reproduces the qualitative features of the magnetic response with regard to the ring size and bonding pattern of C2N rings (see ESI,† Fig. S2 and S3). Chemical shieldings were dissected to contributions from canonical MOs (CMOs) employing the NMR and EPR modules54 of the ADF and the contributions of πout and πin sets of orbitals were constructed by the summation of the corresponding CMOs.
For the calculation of the induced magnetic field the molecules were placed on xy Cartesian plane and the chemical shielding was calculated in a 313 cubic grid of points with a separation step of 0.5 Å. Extraction of CMO contributions at each point and generation of cube files per MO were performed with our custom MIMAF code.55 Visualization of induced magnetic field isosurfaces was performed with the VMD.56
Results and discussion
Geometrical characteristics
The ground state optimized geometries of C4n+2 (n = 1–6) and C4n (n = 2–6) molecules are shown in Fig. S1 (ESI†) and their corresponding geometrical parameters are given in Table 1. All molecules adopt C(n/2)h point group symmetry representing polyynic structures with altering bond lengths and altering bond angles, except for C6 and C10 which adopt D(n/2)h cumulenic structures with equal bond lengths and altering angles. The bond length alteration (BLA) ranges from 0.123 Å to 0.148 Å for all C(n/2)h point group molecules, except for C14 which presents a minimal BLA of 0.051 Å. On the other hand, bond angle alteration (BAA) is very large for small rings starting from 58.4° and 55.6° for C6 and C8, respectively, and decreases rapidly as the ring size increases, reaching a constant value of 1.7° for C2N (N = 11–14), while the smallest BAA (0.4°) is observed in C18. For C4n molecules D(n/2)h geometries with altering bond lengths and equal bond angles were found to be first-order saddle points, whereas higher symmetry Dnh geometries with equal bonds and angles were found to be higher-order saddle points for both C4n+2 and C4n molecules.| Molecule | Point Group | d 1 (Å) | d 2 (Å) | BLAa | θ 1 (°) | θ 2 (°) | BAAb |
|---|---|---|---|---|---|---|---|
| a Bond length alteration, d1 − d2. b Bond angle alteration, θ1 − θ2. | |||||||
| C6 | D 3h | 1.321 | 0.00 | 149.2 | 90.8 | 58.4 | |
| C8 | C 4h | 1.384 | 1.251 | 0.133 | 162.8 | 107.2 | 55.6 |
| C10 | D 5h | 1.290 | 1.290 | 0.00 | 165.0 | 123.0 | 42.1 |
| C12 | C 6h | 1.359 | 1.234 | 0.126 | 168.3 | 131.7 | 36.6 |
| C14 | C 7h | 1.307 | 1.256 | 0.051 | 167.0 | 141.6 | 25.4 |
| C14 TS | D 7h | 1.281 | 0.00 | 168.1 | 140.5 | 27.6 | |
| C16 | C 8h | 1.364 | 1.216 | 0.148 | 158.9 | 156.1 | 2.8 |
| C18 | C 9h | 1.346 | 1.223 | 0.123 | 160.2 | 159.8 | 0.4 |
| C18 TS | D 9h | 1.277 | 0.00 | 169.4 | 150.6 | 18.8 | |
| C20 | C 10h | 1.358 | 1.216 | 0.141 | 162.6 | 161.4 | 1.2 |
| C22 | C 11h | 1.351 | 1.219 | 0.132 | 164.5 | 162.8 | 1.7 |
| C22 TS | D 11h | 1.276 | 0.00 | 171.0 | 156.3 | 14.8 | |
| C24 | C 12h | 1.355 | 1.216 | 0.139 | 165.8 | 164.2 | 1.7 |
| C26 | C 13h | 1.352 | 1.217 | 0.135 | 167.0 | 165.3 | 1.7 |
| C26 TS | D 13h | 1.276 | 0.00 | 172.7 | 159.6 | 13.1 | |
| C28 | C 14h | 1.354 | 1.216 | 0.138 | 168.0 | 166.3 | 1.7 |
For C4n+2 (n = 3–6) molecules, QST2 and IRC calculations generated cumulenic geometries with equal bond lengths and altering bond angles, adopting D(n/2)h point group symmetry, as transition states between two inversion related polyynic ground states28 (Fig. 2). The transition states display increased BAA with regard to the respective ground states. The energy barrier for the inversion increases with the number of carbon atoms, starting from a marginal barrier of 0.12 kcal mol−1 for C14 and reaching 10.90, 24.42 and 37.54 kcal mol−1 for C18, C22 and C26 respectively.
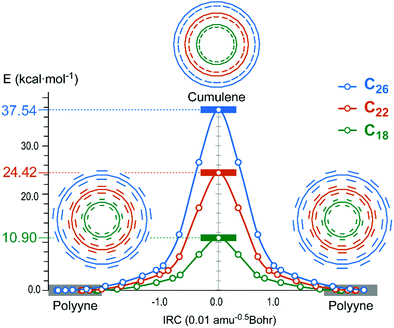 | ||
| Fig. 2 Relative IRC profiles of C18, C22 and C26 depicting the interchange of single–triple bonds of polyynic ground states through a cumulenic transition state. | ||
Total magnetic response
When an (anti)aromatic molecule is subjected to a uniform external magnetic field![[B with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0042_20d1.gif) ext, then closed circuits of mobile electrons induce a current density, which in turn induces a secondary magnetic field,
ext, then closed circuits of mobile electrons induce a current density, which in turn induces a secondary magnetic field, ![[B with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0042_20d1.gif) ind, either opposing (shielding) the external field in aromatic rings or reinforcing (deshielding) the external field in antiaromatic rings. In planar structures the molecule is oriented such that the molecular plane (xy) is perpendicular to the external field's direction (z), and hence the induced magnetic field is designated as
ind, either opposing (shielding) the external field in aromatic rings or reinforcing (deshielding) the external field in antiaromatic rings. In planar structures the molecule is oriented such that the molecular plane (xy) is perpendicular to the external field's direction (z), and hence the induced magnetic field is designated as 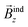 . The induced magnetic field is typically visualized as isosurfaces of Bindz, forming long range shielding or deshielding cones in aromatic or antiaromatic rings respectively. The value of Bindz isosurface at any point is equal to the zz tensor component of nucleus independent chemical shift, NICSzz.
. The induced magnetic field is typically visualized as isosurfaces of Bindz, forming long range shielding or deshielding cones in aromatic or antiaromatic rings respectively. The value of Bindz isosurface at any point is equal to the zz tensor component of nucleus independent chemical shift, NICSzz.
NICSzz values, presented in Table 2 and Fig. S2 (ESI†), reveal the characteristic features of carbon rings’ magnetic response. These features are as follows: (a) C4n+2 molecules display large diatropic (negative) NICSzz representative of aromatic character, whereas C4n molecules display large paratropic (positive) NICSzz representative of antiaromatic character; (b) NICSzz of polyynic C4n+2 (n = 3–6) and C4n molecules decreases significantly as the ring size increases; (c) NICSzz of the cumulenic C4n+2 (n = 1–6) geometries increases with the ring size, showing a trend to reach a maximum value at larger rings; (d) accordingly for C4n+2 molecules there is an increasing difference in NICSzz between polyynic ground states and cumulenic transition states with an increment in the ring size; (e) the maximum NICSzz among ground state C4n+2 molecules is observed in C14 which presents the minimum BLA.
| Molecule | Total | πout | πin | Molecule | Total | πout | πin |
|---|---|---|---|---|---|---|---|
| C6 | −37.7 | −39.8 | 19.2 | C8 | 130.9 | 110.5 | 28.2 |
| C10 | −65.2 | −52.1 | −3.3 | C12 | 96.8 | 64.8 | 34.1 |
| C14 | −81.6 | −49.9 | −25.4 | C16 | 57.9 | 27.0 | 31.7 |
| C14 TS | −89.2 | −56.7 | −25.0 | C20 | 33.4 | 15.5 | 18.7 |
| C18 | −44.7 | −20.6 | −16.4 | C24 | 18.7 | 8.6 | 10.8 |
| C18 TS | −101.5 | −58.3 | −37.2 | C28 | 10.5 | 4.8 | 6.4 |
| C22 | −22.0 | −10.7 | −7.8 | C8H8 | 107.2 | 90.2 | |
| C22 TS | −104.0 | −58.1 | −40.7 | ||||
| C26 | −10.9 | −5.4 | −3.4 | ||||
| C26 TS | −99.3 | −55.8 | −39.0 | ||||
| C6H6 | −15.9 | −36.7 | |||||
| C18H18 | −41.5 | −48.0 | |||||
| C18H6 | −8.1 | −12.7 | −15.7 | ||||
| B9H9 | 3.3 | 0.5 | 3.4 |
The above remarks clarify controverting NICS studies of C4n+2 rings.19,20 The NICS values reported by Fowler et al. show an increment in diatropic NICS of C4n+2 for n = 1–5 and then a significant decrement for n = 6, 7 due to molecular geometry obtained with B3LYP functional which predicts cumulenic structures for n = 1–5 and polyynic structures for n = 6, 7.19 On the other hand, Suresh and Remya reported increasing diatropic NICS values for all C4n+2 (n = 1–7) trending to a maximum value due to cumulenic geometries obtained with MO6L functional.20
Visualizations of Bindz induced by all electrons of C2N molecules are presented in Fig. 3 using multiple clipped isosurfaces (±5 ppm and ±20 ppm) to depict the long-range effect, as well as a full isosurface of large Bindz values to illustrate specific characteristics of the magnetic response close to the molecular domain. C4n+2 molecules induce strong long-range shielding cones representative of strong aromatic character. The long-range shielding cone (cyan, −5 ppm) of C4n+2 molecules increases with the ring size but the actual trend is revealed by inspection of the strong response close to the molecular plane depicted with green isosurfaces (−50 ppm) in Fig. 3. In ground state polyynic structures the extension of the short-range response increases from C10 to C14 where it reaches a maximum span forming a uniform shielding cone, and then gradually decreases from C18 to C26, deforming to a toroidal shape inside the ring of C26. On the contrary the strong shielding cone of cumulenic transition states retains its uniform long range shape. Therefore, the weakening of the magnetic response in ground states is attributable to polyynic bonding and is irrelevant to the ring size. However, the discrepancy in the magnetic response between ground and transition states for C18, C22 and C26 is apparently evolving with the ring size. Thus, according to the magnetic criterion, the aromaticity of polyynic C4n+2 decreases for n > 3 while it is retained for the respective cumulenic transition states.
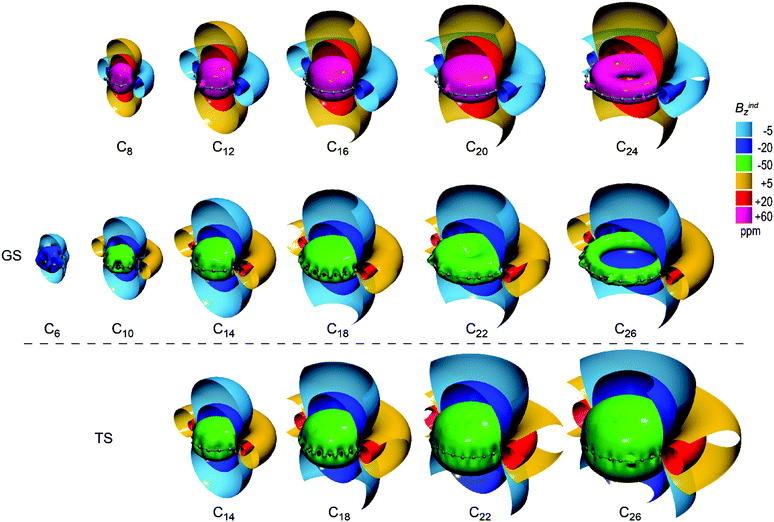 | ||
| Fig. 3 Isosurfaces of total Bindz of transition states D(n/2)h C4n+2 (n = 3–6) (bottom row), ground states C(n/2)h C4n+2 (n = 1–6) (middle row) and C4n (n = 2–6) (top row). | ||
C4n molecules sustain strong long-range deshielding cones representative of strong antiaromatic character. However strong short-range response (+60 ppm) decreases with the evolution of ring size, starting with extended uniform deshielding cones in C8 and C12 and downgrading to a toroidal shape in C24, which implies the weakening of antiaromaticity.
Magnetic responses of πout and πin orbitals
The total magnetic response suggests the (anti)aromatic character of C2N carbon rings but does not give any clear information about the source of the long range (de)shielding cones and the individual role of each (para)diatropic circuit in the double aromatic character, because it is constructed by contributions from all πout, πin, σ and core orbitals. In order to gain insight into the origins and the duality of the (anti)aromatic character of C2N carbon rings, we dissected the magnetic response to contributions from πout and πin sets of orbitals, which are exclusive sources of the strong long-range magnetic response.In Fig. 4, the respective magnetic responses of πout and πin sets of orbitals of ground states C4n+2 molecules are given. It is clear that for C14 and larger rings, both πout and πin orbitals induce long range shielding cones, illustrating the dual source of their diatropicity. Smaller members C6 and C10 display no or a very weak in-plane magnetic response, respectively, due to a large bond angle alteration (BAA) which leads to a small in-plane orbital overlap. Specifically, the πout orbitals of C6 induce a shielding cone very similar to benzene and have comparable πout–NICSzz values (−39.8 and −36.7 ppm for C6 and C6H6 respectively). The πin orbitals of C6 induce a weak short range diatropic response with a paratropic sphere inside the ring (+19.2 ppm at ring center), which is representative of localized CC σ bonding.43 Therefore, C6 displays equal diatropicity with benzene originating from the out-of-plane π system, and the large difference in their total NICSzz does not arise from delocalized electrons but from localized σ bonds which have larger paratropic contributions in benzene. C10 presents a strong long-range shielding cone induced from πout orbitals with −52.1 ppm contributions at the ring center, and a considerably weaker shielding cone induced from πin orbitals with only −3.3 ppm at the ring center, denoting a marginal in-plane π magnetic response due to a large BAA.
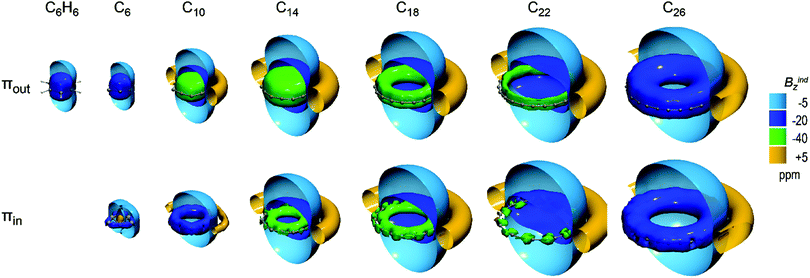 | ||
| Fig. 4 Isosurfaces of πout (top) and πin (bottom) contributions to Bindz of polyynic ground states of C(n/2)h C4n+2 (n = 1–6) rings. | ||
C14 induces long range shielding cones from both πout and πin orbitals but the out-of-plane response is much stronger than that of the in-plane, as πout forms an extended uniform −40 ppm isosurface, while πin forms a condensed shielding toroid inside the ring and the πout–NICSzz (−49.9 ppm) is the double of πin–NICSzz (−25.4 ppm). This difference is attributed to the geometry of C14, which presents the minimum BLA that favors the out-of-plane delocalization and significant BAA that hinders the in-plane delocalization. The corresponding transition state displays an almost identical magnetic response, both in terms of shielding cones and NICSzz values, due to small structural differences in the two states.
For the ground states of larger rings C18, C22 and C26, πout and πin display very similar shielding cones. The πin–NICSzz is only 2–4 ppm less diatropic than πout–NICSzz, denoting a marginally increased out-of-plane magnetic response. The diatropicity decreases with an increment in the ring size for both sets of orbitals. Specifically NICSzz of πout (πin) decrease from −20.6 (−16.4) ppm in C18 down to −10.7 (−7.8) and −5.4 (−3.4) ppm in C22 and C26 respectively.
In order to assess the C18 diatropicity we used as reference three prototypical molecules with 18-π electrons and varying degrees of aromaticity, namely 18-annulene, 18-dehydroannulene and the BN analogue57 B9N9, as presented in Fig. 5. Compared to the π response of 18-annulene (Fig. 5b), the πout of C18 induces a weaker shielding cone and presents less than half NICSzz, denoting a considerable weaker out-of-plane magnetic response in C18. If we consider both πout and πin orbitals of C18, their cumulative shielding cone (Fig. 5a) is greater than Bindπz of 18-annulene, but this is due to the overestimation of PBE which predicts a total (πout + πin)–NICSzz value 21 ppm to be more diatropic than NICSπzz of C18H18, while with BHandHLYP it is 11.0 ppm less diatropic. Therefore, C18 displays a similar or a slightly weaker diatropicity when compared to C18H18. Furthermore, 18-dehydroannule (Fig. 5c) is less aromatic than C18 and induces a weaker πout shielding cone (πout–NICSzz is −12.7 ppm), as well as a weak short-range paratropic response from 12 πin electrons (πin–NICSzz is +15.7 ppm). Finally, B9N9 (Fig. 5d) is a characteristic example with extreme localized electrons, with both 18 πout and 18 πin electrons inducing weak short range diatropic cones strictly positioned on nitrogen atoms. Hence C4n+2 (n > 3) carbon rings, although they exhibit dual source of diatropicity, show that their magnetic responses are comparable to those of classical π-aromatic annulenes.
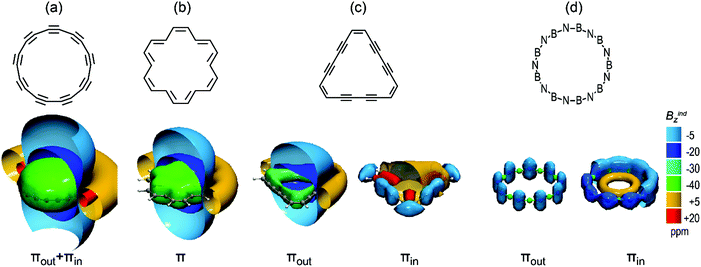 | ||
| Fig. 5 Isosurfaces of π contributions to Bindz of (a) C9h C18, (b) 18-annulene, (c) 18-dehydroannulene and (d) B9N9. | ||
The magnetic responses of transition states, presented in Fig. 6, display constant strong shielding cones of both πout and πin. Specifically, πout–NICSzz retains a value of around −57 ppm, whereas πin–NICSzz amounts to ∼ −39 ppm for all transition states, implying favorable πout delocalization. The difference in πout and πin can be explained by the increased BAA of cumulenic structures which hinders the in-plane overlap. Indeed, the third-order saddle point D18h geometry of C18 with zero BLA and BAA presents almost equal πout and πin NICSzz values (−60.2 and −58.7 ppm respectively). However, πin electrons in cumulenic geometries are still more diatropic than polyynic. Hence BLA is the main factor that constrains both πout and πin magnetic responses and BAA affects secondarily only the πin magnetic response. Consequently, the cumulenic geometries are very diatropic from both sources of magnetic response.
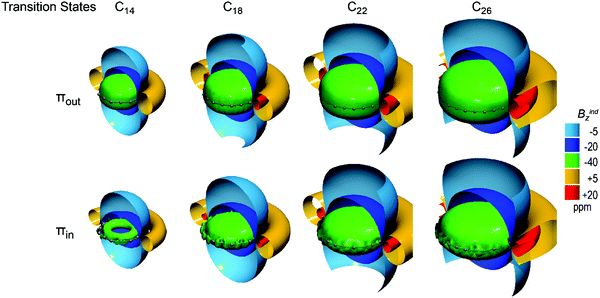 | ||
| Fig. 6 Isosurfaces of πout (top) and πin (bottom) contributions to Bindz of cumulenic transition states of D(n/2)h C4n+2 (n = 3–6) rings. | ||
Concerning the antiaromatic C4n (n = 2–6) rings, both πout and πin orbitals induce equivalent long range deshielding cones (Fig. 7), except for C8 which exhibits only πout deshielding cone and a weak short range paratropic response of πin due to large BAA, which does not justify a paratropic in-plane ring current. The πin response of C14 is also weak due to large BAA but still forms a deshielding cone denoting weak in-plane paratropic current, whereas for larger rings πout and πin induce equivalent deshielding cones. The πout deshielding cones of C8 and C12 are comparable to that of planar C8H8 but decline significantly for larger rings, as the πin deshielding cones also do. Hence, C4n (n = 3–6) rings exhibit a dual source of paratropicity representative of antiaromatic character which weakens significantly with an increment in the ring size.
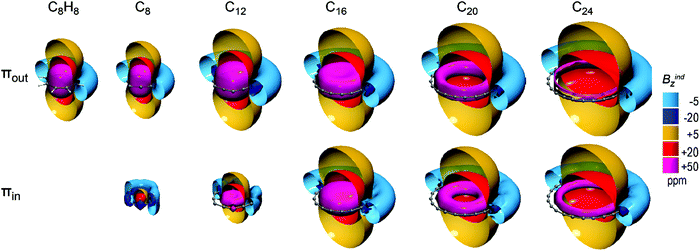 | ||
| Fig. 7 Isosurfaces of πout (top) and πin (bottom) contributions to Bindz of C4n (n = 2–6) rings and planar C8H8. | ||
CMO contributions
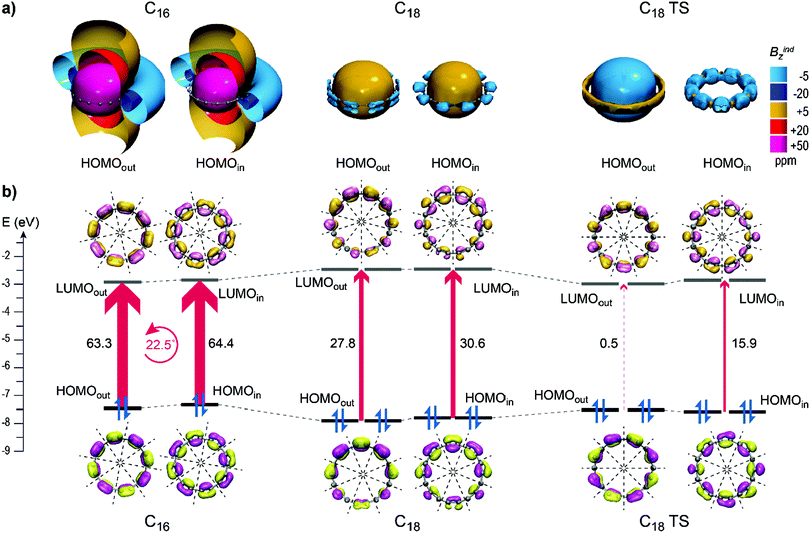
In order to elucidate the above remarks, we have to take into consideration the electronic structure of carbon rings, especially the frontier orbitals that principally determine the overall magnetic response. Dissection of the induced magnetic field to CMO contributions reveals that lower energy valence orbitals induce a diatropic response, whereas the highest occupied orbitals dictate the overall magnetic response.43,44 Indeed in C2N, the contributions of πout and πin to NICSzz are linearly correlated to the contribution of the corresponding HOMOs (R2 = 0.97, Fig. S4, ESI†). Hence the (para)diatropicity can be analyzed in terms of HOMO contributions, as given in Table S4 and Fig. S5, S6 (ESI†).Generally, in antiaromatic molecules the HOMOs induce a very strong paratropic response that overwhelms the diatropicity of lower orbitals, whereas in aromatic molecules the HOMOs induce a very weak paratropic or diatropic response that adjusts the overall diatropicity and tunes the aromaticity. In turn, the paratropic response of HOMOs originates from symmetry allowed rotational excitations to unoccupied orbitals and its magnitude depends on the energy gap and the overlap of interacting orbitals.43,44,46,58–60
In C4n rings, the HOMO and LUMO of both out-of-plane and in-plane orientations are non-degenerate MOs with the same number of n nodal planes and the same symmetry. Hence the symmetry allowed HOMOout/in → LUMOout/in excitations, rotating the HOMOs by an angle of 2π/4n, to lead to an optimum overlap producing maximum paratropic response. For example, in C16 the rotational excitations of HOMOout and HOMOin with 4 nodal planes rotated by 22.5° lead to a perfect overlap with LUMOout and LUMOin contributing with +63.3 and +64.4 ppm, respectively, to NICSzz (Fig. 8b) and induce very strong long range deshielding cones (Fig. 8a) that dominate on the overall magnetic response. As the HOMO–LUMO gaps remain practically unchanged throughout the C4n series (Table S4, ESI†), the decline in paratropicity originates from a weakening paratropic response of HOMOs and is attributed to the decrease in rotational overlap as the number of nodal planes increases.
In C4n+2 rings, both HOMOout and HOMOin are doubly degenerate with n nodal planes, whereas the LUMOs are doubly degenerate with n + 1 nodal planes. In a perfect symmetry of Dnh point group a HOMO → LUMO rotational excitation would be symmetry forbidden, but in D(n/2)h and C(n/2)h point groups such excitations are symmetry allowed. However due to a different number of nodal planes the overlap is small, inducing a weak magnetic response that depends on the geometrical characteristics of the ring. For example in C9h C18, the frontier orbitals are distorted due to significant BLA and the HOMO → LUMO excitations display a small overlap inducing weak paratropic response (Fig. 8a) that diminishes the diatropicity of the lower energy orbitals, contributing with +27.8 and +30.6 ppm to NICSzz of πout and πin, respectively (Fig. 8b). In contrast, in D9h C18 the zero BLA leads to a negligible rotational overlap of HOMOout → LUMOout excitation, resulting in the diatropic response of HOMOout that adds to the overall diatropicity. On the other hand, HOMOin → LUMOin excitations of D9h C18 induce small paratropic contributions (+15.9 ppm) due to increased BAA that leads to a weaker diatropicity of πin with regard to πout. The same holds for the ground states of smaller members C6, C10 and C14 with zero (or almost zero) BLA, which induce shielding cones from HOMOout (Fig. S5, ESI†) and display increased πout diatropicity. For C4n+2 ground states the increment in HOMO nodal planes with the ring size results in a gradual increment in the small overlap and hence in the augmentation of HOMO paratropic contributions (Fig. S5, ESI†), causing a gradual decrement in the overall diatropicity.
Conclusions
The dissection of the induced magnetic field to contributions from out-of-plane and in-plane π orbitals revealed the dual aromatic and antiaromatic character of C4n+2 and C4n rings, respectively, according to the magnetic criterion. The magnetic response induced from distinctive sets of electrons is sensitive to the bonding pattern and geometrical features, where polyynic C4n+2 ground states display similar out-of-plane and in-plane magnetic responses and comparable diatropicity to annulenes, while the cumulenic transition states exhibit a very strong diatropic character and an augmented out-of-plane magnetic response with regard to the in-plane magnetic response. Bond length alteration is the prime factor that constrains both πout and πin magnetic responses, whereas the bond angle alteration affects secondarily only the πin magnetic response. A significant decline in magnetic response with an increment in the ring size is observed for both ground state aromatic and antiaromatic rings, which is attributed to decreasing paratropic contributions of HOMOs originating from rotational excitations.Conflicts of interest
There are no conflicts to declare.References
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl and R. E. Smalley, C60: Buckminsterfullerene, Nature, 1985, 318, 162–163 CrossRef CAS.
- K. S. Novoselov, Electric Field Effect in Atomically Thin Carbon Films, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- S. Iijima, Helical microtubules of graphitic carbon, Nature, 1991, 354, 56–58 CrossRef CAS.
- Y. Wang, P. Yang, L. Zheng, X. Shi and H. Zheng, Carbon nanomaterials with sp2 or/and sp hybridization in energy conversion and storage applications: A review, Energy Storage Mater., 2020, 26, 349–370 CrossRef.
- M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Carbon nanotubes: Present and future commercial applications, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- L. Bacakova, J. Pajorova, M. Tomkova, R. Matejka, A. Broz, J. Stepanovska, S. Prazak, A. Skogberg, S. Siljander and P. Kallio, Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine, Nanomaterials, 2020, 10, 196 CrossRef CAS PubMed.
- J. L. Blackburn, A. J. Ferguson, C. Cho and J. C. Grunlan, Adv. Mater., 2018, 30, 1704386 CrossRef PubMed.
- B. Han, Y. L. Zhang, Q. D. Chen and H. B. Sun, Adv. Funct. Mater., 2018, 28, 1802235 CrossRef.
- N. Panwar, A. M. Soehartono, K. K. Chan, S. Zeng, G. Xu, J. Qu, P. Coquet, K. T. Yong and X. Chen, Chem. Rev., 2019, 119, 9559–9656 CrossRef CAS PubMed.
- A. Rajkamal and R. Thapa, Adv. Mater. Technol., 2019, 4, 1900307 CrossRef CAS.
- Y. Jiao, A. Du, M. Hankel and S. C. Smith, Modelling carbon membranes for gas and isotope separation, Phys. Chem. Chem. Phys., 2013, 15(14), 4832–4843 RSC.
- P. Avouris, M. Radosavljević and S. Wind, Carbon Nanotube Electronics and Optoelectronics, in Applied Physics of Carbon Nanotubes, ed. S. V. Rotkin and S. Subramoney, NanoScience and Technology, Springer, Berlin, Heidelberg, 2005 Search PubMed.
- R. H. Baughman, H. Eckhardt and M. Kertesz, Structure-property predictions for new planar forms of carbon: Layered phases containing sp2 and sp atoms, J. Chem. Phys., 1987, 87, 6687–6699 CrossRef CAS.
- F. Diederich, Y. Rubin, C. B. Knobler, R. L. Whetten, K. E. Schriver, K. N. Houk and Y. Li, All-Carbon Molecules: Evidence for the Generation of Cyclo[18]carbon from a Stable Organic Precursor, Science, 1989, 245, 1088–1090 CrossRef CAS PubMed.
- X. Gao, H. Liu, D. Wang and J. Zhang, Graphdiyne: synthesis, properties, and applications, Chem. Soc. Rev., 2019, 48, 908–936 RSC.
- C. Xie, N. Wang, X. Li, G. Xu and C. Huang, Research on the Preparation of Graphdiyne and Its Derivatives, Chem. – Eur. J, 2020, 26, 569–583 CrossRef CAS PubMed.
- R. Sakamoto, N. Fukui, H. Maeda, R. Matsuoka, R. Toyoda and H. Nishihara, The Accelerating World of Graphdiynes, Adv. Mater., 2019, 31, 1804211 CrossRef CAS PubMed.
- K. Kaiser, L. M. Scriven, F. Schulz, P. Gawel, L. Gross and H. L. Anderson, An sp-hybridized molecular carbon allotrope, cyclo[18]carbon, Science, 2019, 365, 1299–1301 CrossRef CAS PubMed.
- P. W. Fowler, N. Mizoguchi, D. E. Bean and R. W. A. Havenith, Double Aromaticity and Ring Currents in All-Carbon Rings, Chem. – Eur. J., 2009, 15, 6964–6972 CrossRef CAS PubMed.
- K. Remya and C. H. Suresh, Carbon rings: a DFT study on geometry, aromaticity, intermolecular carbon–carbon interactions and stability, RSC Adv., 2016, 6, 44261–44271 RSC.
- C. Neiss, E. Trushin and A. Görling, The Nature of One-Dimensional Carbon: Polyynic versus Cumulenic, ChemPhysChem, 2014, 15, 2497–2502 CrossRef CAS PubMed.
- M. Feyereisen, M. Gutowski, J. Simons and J. Almlöf, Relative stabilities of fullerene, cumulene, and polyacetylene structures for Cn: N = 18–60, J. Chem. Phys., 1992, 96, 2926–2932 CrossRef CAS.
- V. Parasuk, J. Almlof and M. W. Feyereisen, The [18] all-carbon molecule: cumulene or polyacetylene?, J. Am. Chem. Soc., 1991, 113, 1049–1050 CrossRef CAS.
- D. A. Plattner and K. N. Houk, C18 Is a Polyyne, J. Am. Chem. Soc., 1995, 117, 4405–4406 CrossRef CAS.
- T. Torelli and L. Mitas, Electron Correlation in C4N + 2 Carbon Rings: Aromatic versus Dimerized Structures, Phys. Rev. Lett., 2000, 85, 1702–1705 CrossRef CAS PubMed.
- S. Arulmozhiraja and T. Ohno, CCSD calculations on C14, C18, and C22 carbon clusters, J. Chem. Phys., 2008, 128, 114301 CrossRef PubMed.
- A. J. Stasyuk, O. A. Stasyuk, M. Solà and A. A. Voityuk, Cyclo[18]carbon: the smallest all-carbon electron acceptor, Chem. Commun., 2020, 56, 352–355 RSC.
- G. V. Baryshnikov, R. R. Valiev, A. V. Kuklin, D. Sundholm and H. Ågren, Cyclo[18]carbon: Insight into Electronic Structure, Aromaticity, and Surface Coupling, J. Phys. Chem. Lett., 2019, 10, 6701–6705 CrossRef CAS PubMed.
- É. Brémond, Á. J. Pérez-Jiménez, C. Adamo and J. C. Sancho-García, Sp-hybridized carbon allotrope molecular structures: An ongoing challenge for density-functional approximations, J. Chem. Phys., 2019, 151, 211104 CrossRef PubMed.
- A. Nandi, E. Solel and S. Kozuch, Carbon Tunneling in the Automerization of Cyclo[18]carbon, Chem. – Eur. J., 2020, 26, 625–628 CrossRef CAS PubMed.
- R. Hoffmann, Chemical Abstracts Service: Cumulenes, polyenes, polyacetylenes and cn, Tetrahedron, 1966, 22, 521–538 CrossRef CAS.
- M. D. Wodrich, C. Corminboeuf, S. S. Park and P. Von Ragué Schleyer, Double aromaticity in monocyclic carbon, boron, and borocarbon rings based on magnetic criteria, Chem. – Eur. J., 2007, 13, 4582–4593 CrossRef CAS PubMed.
- R. Gershoni-Poranne and A. Stanger, Magnetic criteria of aromaticity, Chem. Soc. Rev., 2015, 44, 6597–6615 RSC.
- M. K. Cyrański, Energetic Aspects of Cyclic Pi-Electron Delocalization: Evaluation of the Methods of Estimating Aromatic Stabilization Energies, Chem. Rev., 2005, 105, 3773–3811 CrossRef PubMed.
- J. Poater, M. Duran, M. Solà and B. Silvi, Theoretical Evaluation of Electron Delocalization in Aromatic Molecules by Means of Atoms in Molecules (AIM) and Electron Localization Function (ELF) Topological Approaches, Chem. Rev., 2005, 105, 3911–3947 CrossRef CAS PubMed.
- D. W. Szczepanik, M. Solà, M. Andrzejak, B. Pawełek, J. Dominikowska, M. Kukułka, K. Dyduch, T. M. Krygowski and H. Szatylowicz, The role of the long-range exchange corrections in the description of electron delocalization in aromatic species, J. Comput. Chem., 2017, 38, 1640–1654 CrossRef CAS PubMed.
- G. Merino, T. Heine and G. Seifert, The Induced Magnetic Field in Cyclic Molecules, Chem. – Eur. J., 2004, 10, 4367–4371 CrossRef CAS PubMed.
- R. Islas, T. Heine and G. Merino, The Induced Magnetic Field, Acc. Chem. Res., 2012, 45, 215–228 CrossRef CAS PubMed.
- R. Gershoni-Poranne and A. Stanger, The NICS- XY -Scan: Identification of Local and Global Ring Currents in Multi-Ring Systems, Chem. – Eur. J., 2014, 20, 5673–5688 CrossRef CAS PubMed.
- R. Gershoni-Poranne and A. Stanger, Magnetic criteria of aromaticity, Chem. Soc. Rev., 2015, 44, 6597–6615 RSC.
- I. G. Cuesta, A. S. De Merás, S. Pelloni and P. Lazzeretti, Understanding the ring current effects on magnetic shielding of hydrogen and carbon nuclei in naphthalene and anthracene, J. Comput. Chem., 2009, 30, 551–564 CrossRef CAS PubMed.
- R. Benassi, P. Lazzeretti and F. Taddei, Magnetic criteria for aromaticity, J. Phys. Chem., 1975, 79, 848–851 CrossRef CAS.
- N. D. Charistos, A. G. Papadopoulos and M. P. Sigalas, Interpretation of Electron Delocalization in Benzene, Cyclobutadiene, and Borazine Based on Visualization of Individual Molecular Orbital Contributions to the Induced Magnetic Field, J. Phys. Chem. A, 2014, 118, 1113–1122 CrossRef CAS PubMed.
- N. D. Charistos, A. G. Papadopoulos, T. A. Nikopoulos, A. Muñoz-Castro and M. P. Sigalas, Canonical orbital contributions to the magnetic fields induced by global and local diatropic and paratropic ring currents, J. Comput. Chem., 2017, 38, 2594–2604 CrossRef CAS PubMed.
- N. D. Charistos and A. Muñoz-Castro, Double aromaticity of the B 40 fullerene: induced magnetic field analysis of π and σ delocalization in the boron cavernous structure, Phys. Chem. Chem. Phys., 2019, 21, 20232–20238 RSC.
- N. D. Charistos, A. Muñoz-Castro and M. P. Sigalas, The pseudo-π model of the induced magnetic field: fast and accurate visualization of shielding and deshielding cones in planar conjugated hydrocarbons and spherical fullerenes, Phys. Chem. Chem. Phys., 2019, 21, 6150–6159 RSC.
- J. Da Chai and M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- A. Ehnbom, M. B. Hall and J. A. Gladysz, Origin of Shielding and Deshielding Effects in NMR Spectra of Organic Conjugated Polyynes, Org. Lett., 2019, 21, 753–757 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, Chemistry with ADF, J. Comput. Chem., 2001, 22, 931–967 CrossRef CAS.
- ADF, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, 2019, http://www.scm.com Search PubMed.
- A. D. Becke, A new mixing of Hartree-Fock and local density-functional theories, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- G. Schreckenbach and T. Ziegler, Calculation of NMR Shielding Tensors Using Gauge-Including Atomic Orbitals and Modern Density Functional Theory, J. Phys. Chem., 1995, 99, 606–611 CrossRef CAS.
- N. Charistos, T. Nikopoulos and M. Sigalas, MIMAF (Molecular Induced MAgnetic Fields) code, Laboratory of Quantum and Computational Chemistry, Department of Chemistry, Aristotle University of Thessaloniki, Thessaloniki, 2016 Search PubMed.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- F. Pichierri, Boron-nitrogen analogues of cyclo[18]carbon, Chem. Phys. Lett., 2020, 738, 136860 CrossRef.
- C. Corminboeuf, R. B. King and P. von R. Schleyer, Implications of Molecular Orbital Symmetries and Energies for the Electron Delocalization of Inorganic Clusters, ChemPhysChem, 2007, 8, 391–398 CrossRef CAS PubMed.
- I. Pérez-Juste, M. Mandado and L. Carballeira, Contributions from orbital–orbital interactions to nucleus-independent chemical shifts and their relation with aromaticity or antiaromaticity of conjugated molecules, Chem. Phys. Lett., 2010, 491, 224–229 CrossRef.
- C. M. Widdifield and R. W. Schurko, Understanding chemical shielding tensors using group theory, MO analysis, and modern density-functional theory, Concepts Magn. Reson. Part A, 2009, 34A, 91–123 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cp01252a |
| This journal is © the Owner Societies 2020 |