Rate constants and kinetic isotope effects for H-atom abstraction reactions by muonium in the Mu + propane and Mu + n-butane reactions from 300 K to 435 K: challenges for theory
Abstract
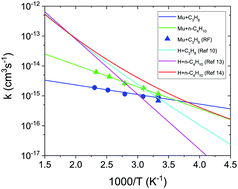
This paper reports measurements of the temperature dependence of the rate constants for H-atom abstraction reactions from propane and n-butane by the light isotopic H-atom muonium (Mu), kMu(T), over temperatures in the range 300 K to 435 K. Simple Arrhenius fits to these data yield activation energies, EMua, that are some 2–4 times lower than EHa found from corresponding fits for the H + propane and H + n-butane reactions studied elsewhere, both experimentally and theoretically, and fit over a similar temperature range. These activation energies EMua are also much lower than estimated from zero-point-energy corrected vibrationally adiabatic potential barriers, both results suggesting that quantum tunneling plays an important role in determining kMu(T) and for the Mu + propane reaction in particular. The results are expected to pose a considerable challenge to reaction rate theory for isotopic H-atom reactions in alkane systems.

 Please wait while we load your content...
Please wait while we load your content...