DFT insights into electrocatalytic CO2 reduction to methanol on α-Fe2O3(0001) surfaces†
Abstract
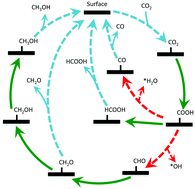
Electrocatalytic reduction of CO2 to manufacture fuels and other useful chemicals is one of the appealing methods to reuse CO2. Herein, electrocatalytic CO2 reduction on a model α-Fe2O3(0001) surface catalyst has been investigated by means of density functional theory. This systematic study, involving 20 reaction intermediates and 63 distinct elementary reaction steps, has allowed the identification of a novel mechanism for the decomposition of the key intermediate *COOH. Methanol is the preferred product, with an overpotential of 0.8 V, over carbon monoxide (CO), formic acid (HCOOH), and formaldehyde (CH2O). Formaldehyde formed on the surface will be converted into methanol. This work demonstrates the need for a complete investigation of possible pathways to find the most favourable one, beyond chemical intuition. Moreover, it suggests that hematite could be an interesting material for CO2 reduction.
- This article is part of the themed collection: Frontiers in Molecular Simulation of Solvated Ions, Molecules and Interfaces


 Please wait while we load your content...
Please wait while we load your content...