 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Is basicity the sole criterion for attaining high carbon dioxide capture in deep-eutectic solvents?†
Shashi Kant
Shukla
 *a,
Dariush
Nikjoo
b and
Jyri-Pekka
Mikkola
*ac
*a,
Dariush
Nikjoo
b and
Jyri-Pekka
Mikkola
*ac
aTechnical Chemistry, Department of Chemistry, Chemical-Biological Centre, Umeå University, SE-90187 Umeå, Sweden. E-mail: shashi.kant.shukla@umu.se; jyri-pekka.mikkola@umu.se
bDivision of Materials Science, Luleå University of Technology, SE-97187, Luleå, Sweden
cIndustrial Chemistry & Reaction Engineering, Department of Chemical, Engineering, Johan Gadolin Process Chemistry Centre, Åbo Akademi University, FI-20500 Åbo-Turku, Finland
First published on 6th December 2019
Abstract
A critical analysis of the role of Hammett basicity (H−) and aqueous basicity (pKa) in CO2 uptake in deep-eutectic solvents (DESs) suggests that neither H− nor pKa correlates with the CO2 w/w% capacity in the studied DESs. Instead, strong “synergistic interactions” between donor and acceptor moieties satisfactorily relate to the w/w% of CO2 in DESs.
The use of renewable energy as the primary energy supply also requires the improvement in the conservation efficiency of fossil fuels and can only be accomplished by efficient environment-friendly carbon dioxide (CO2) capture.1 The anthropogenic emissions of CO2 in the atmosphere are surging day-by-day and increasing the threat of global warming.2 The current state-of-the-art technology in the CO2 capture is the use of basic amine solutions as a scrubber though this methodology is associated with several critical shortcomings such as high regeneration energy, amine volatility and corrosivity and high maintenance cost of the setup.3,4
The past century has witnessed a lot of effort in quest of “green and sustainable” solvents alternative to volatile organic solvents. Among them, ionic liquids (ILs) and deep-eutectic solvents (DESs) have garnered maximum attention, as they have the potential to be utilized in various applications because of their advantageous properties such as often insignificant vapour pressure, high thermal stability, a wide liquidus range, low flammability and good recyclability.5,6 In the past two decades, interest emerged in testing the ability of both ILs and DESs as scrubber solvents in the acid gas (CO2 and SO2) sequestration.
The pioneering work of Blanchard's et al. showed that CO2 can be partitioned in the IL phase with the least contamination to the CO2 phase.7 This study garnered the attention of researchers worldwide and the last decade has witnessed an upsurge in utilizing ILs in the CO2 capture.8–10 As an example, use of a 60% IL–water mixture instead of aqueous monoethanolamine (MEA) lowered the energy costs by 16%.11
Later, significant improvements in the CO2 capture were noticed upon the introduction of a basic moiety on the cation side-chain and involving a highly basic anion.12–23 However, a direct correlation of CO2 loading (w/w%) with the basicity is missing for ILs. Despite the extensive research efforts in the CO2 capture in ILs, these coulombic solvents turn into a viscous glue and hence render regeneration very difficult.24,25 Furthermore, the synthesis of task-effective ILs is not cost-competitive as it requires several synthetic and purification steps compared to MEA.13,22,26
Contrary to the ILs, DESs, which require a single step atom-economic process and have properties analogous to ILs, provide a promising alternative in CO2 capture and result in a higher gravimetric uptake.27 The efficiency of DESs in CO2 capture relies on complexing an adequate hydrogen bond acceptor (HBA) with an efficient hydrogen bond donor at an optimal molar ratio.28 Because of the ease in terms of property tunability, in a short span, DESs have found application in synthesis, polymerization, stabilization of biomolecules, nanotechnology, separation, extraction of various compounds and so on.29–33 The screening of DESs in CO2 capture showed moderate to high CO2 uptake in potential DESs.34–40 Out of several structural features of DESs, the role of HBD components was acknowledged more in attaining high gravimetric CO2.35–37
Despite the huge success in identifying the potential media for CO2 capture, the major discussion revolves around the acid–base interactions between the solvents and CO2 molecules. Be it the aqueous MEA, methyldiethylamine (MDEA), ionic liquids (ILs) or deep-eutectic solvents (DESs), basicity has been the only criterion suggested for the high CO2 uptake although it failed at some instances.40 The activation of amino-acid ILs by the carboxyl group has shown the importance of the “multiple-site interactions” in multimolar CO2 capture.41 Recently, we discussed the significance of the intermolecular interactions in guiding high CO2 uptake in DESs.42 The “synergistic interaction” between the donor and acceptor moieties in DESs was observed as the controlling parameter in the temperature-dependent CO2 capture in ethylenediamine (EDA)- and polyamine-based DESs.43 Though the multiple-site-, intermolecular- and synergistic-interactions are accounted where basicity does not act as a guiding factor, their applicability, especially the synergistic interactions, which can be represented by making use of the Kamlet–Taft parameters,43 has not been tested as a guiding criterion in the acid gas capture. The current work presents a critical discussion about the role of basicity and synergistic interactions in the CO2 uptake in DESs. The Hammett basicity (H−) and aqueous basicity of HBD components are applied as basicity parameters for DESs.
The DESs employed in the present work were synthesized by complexing monoethanolammonium chloride ([MEA.Cl]), tetrabutylammonium bromide ([TBAB]), and diethylenetriammonium trichloride ([DETA.Cl]) with moderate to high basicity HBDs such as ethanolamine ([EA]), diethanolamine ([DEA]), triethanolamine ([TEA]), [EDA], 3-amino-1-propanol ([AP]) and tetraethylenepentamine ([TEPA]) (see the ESI† for synthesis, entry 1) and their structures are exhibited in Fig. 1. Their characterization results and CO2 uptake monitoring by 13C NMR are given in the ESI† (entries 2–6).
For all the DESs, the CO2 uptake at saturation, Hammett basicity (H−), pseudo-rate constant (k) and some Kamlet–Taft parameters (α and β) are shown in Table 1 and their methods are discussed in the ESI† (see entry 7). ET(30) and π* are given in Table S2 (see the ESI†). For all the DES systems, the mass of CO2 captured was observed to fit into an exponential growth model equation proposed by Box & Lucas (see the ESI,† Fig S34).
| wCO2 = a(1 − e−kt) | (1) |
| DESs | k (min−1) × 102 | CO2 uptake (w/w%) | H − | α | β |
|---|---|---|---|---|---|
| Errors associated with the pseudo rate constant (k), wt% CO2, polarity parameters and Hammett basicity are ±0.003, ±0.004, ±0.004, and, ±0.003, respectively. | |||||
[ChCl][EA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
25.9 | 29.2 | 4.83 | 0.64 | 0.68 |
[ChCl][DEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
14.9 | 19.6 | 4.40 | 0.78 | 0.68 |
[ChCl][TEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
5.0 | 8.0 | 4.37 | 0.81 | 0.68 |
[TBAB][EA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
15.0 | 19.7 | 4.54 | 0.42 | 0.81 |
[TBAB][DEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
4.1 | 9.6 | 4.43 | 0.45 | 0.78 |
[TBAB][TEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3.1 | 2.5 | 4.35 | 0.53 | 0.76 |
[DETA.Cl][EDA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
15.7 | 32.2 | 6.51 | 0.83 | 0.65 |
[DETA.Cl][AP] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
12.4 | 18.3 | 4.43 | 1.03 | 0.61 |
[DETA.Cl][TEPA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2.9 | 9.9 | 5.59 | 0.57 | 1.28 |
For convenience, the dependence of CO2 uptake on the structural parameters of DESs is analyzed in terms of the change in HBD and HBA components. The influence of different HBDs on the CO2 uptake incorporated with similar HBAs is demonstrated in Fig. 2.
 | ||
Fig. 2 CO2 uptake in (a) [ChCl]- and (b) [TBAB]-based DESs containing HBDs [EA] (■), [DEA] ( ) and [TEA] ( ) and [TEA] ( ) whereas (c) in [DETA.Cl]-class of DESs comprising [EDA] (□), [AP] ( ) whereas (c) in [DETA.Cl]-class of DESs comprising [EDA] (□), [AP] ( ) and [TEPA] ( ) and [TEPA] ( ) as HBDs. ) as HBDs. | ||
In [ChCl]- and [TBAB]-classes of DESs, CO2 wt% lowers with the decreasing basicity of HBD from [EA] to [DEA] to [TEA] as shown in Fig. 2(a) and (b). The large difference in the CO2 absorption isotherms in [ChCl]- and [TBAB]-based DESs indicates the influence of HBDs on CO2 capture. Furthermore, the Hammett basicity (k) of DESs and aqueous basicity (pKa) of HBD components were noted as guiding factors in CO2 loading (w/w%) in [ChCl]- and [TBAB]-based DESs.
| [ChCl][EA] (4.83) > [ChCl][DEA] (4.40) > [ChCl][TEA] (4.37). |
| [EA] (9.45) > [DEA] (8.88) > [TEA] (7.77). |
The above inference becomes more evident when discussing the CO2 uptake results in [DETA.Cl]-based DESs with [EDA], [AP] and [TEPA] as HBDs (Fig. 2(c)). In the [DETA.Cl]-class of DESs, the CO2 loading (w/w%) does not follow the order of H− or pKa as shown below.
| [DETA.Cl][EDA] (6.51) > [DETA.Cl][TEPA] (5.59) > [DETA.Cl][AP] (4.43). |
| [EDA] (10.71) > [TEPA] (9.81) > [AP] (4.09). |
As the above arrangements of DESs in terms of H− or pKa do not correspond with CO2 wt%, the basicity appears ineffective here. Furthermore, nearly two-fold higher CO2 uptake is noted in [DETA.Cl][AP] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 than in [DETA.Cl][TEPA] = 1
4 than in [DETA.Cl][TEPA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 despite the lower H− or 1/2pKa value of the former. These observations confirm that high CO2 uptake in these DESs is controlled neither by H− nor pKa and seems to be guided by a rather complex interaction between the HBD and HBA components.
4 despite the lower H− or 1/2pKa value of the former. These observations confirm that high CO2 uptake in these DESs is controlled neither by H− nor pKa and seems to be guided by a rather complex interaction between the HBD and HBA components.
Moreover, the HBA's comparison shows higher CO2 loading in [ChCl]- than in [TBAB]-based DESs despite the similar HBD components (Fig. 3). The large difference in the CO2 uptake capacity of [ChCl] and [TBAB] could be partially due to the steric repulsion caused by the long butyl chain present in the [TBAB] and partly to the higher molar ratio of the HBD components. A crossover point is noticed in the case of [TEA] owing to the viscosity variation during the CO2 capture (Fig. 3(c)).31 Given these observations, it can be conferred that along with the basicity of HBD components, the structural and electronic features of HBAs also play an important role in the CO2 capture.
 | ||
Fig. 3 CO2 uptake in [ChCl] – (⊡) and [TBAB] – ( ) based DESs containing (a) [EA], (b) [DEA] and (c) [TEA] as HBDs. ) based DESs containing (a) [EA], (b) [DEA] and (c) [TEA] as HBDs. | ||
In DESs, both the donor and acceptor lose their identity and result in a new continuum with a different set of solvation properties. Therefore, the inclusion of the solvation properties of DESs, which arises due to the complex donor–acceptor interactions, in the current discussion might help in identifying the governing factors which control the CO2 capture.
The solvation behaviors of DESs and ILs have been perceived in terms of the electronic transition energy (ET(30)) and Kamlet–Taft parameters, which comprise of hydrogen bond donor acidity (α), hydrogen bond acceptor basicity (β) and the polarity index (π*).35 The details about the polarity parameters are discussed in the ESI† (see entry 7).
The Kamlet–Taft parameters (α and β) for different DESs are shown in Table 1. The correlation of polarity parameters with the CO2 uptake data for [TBAB]-based DESs is exhibited in Fig. 4. As shown in Fig. 4(a), high CO2 uptake is favored in less polar DESs. An inverse trend between π* and ET(30) for the [TBAB]-class of DESs is due to the high β value (Fig. 4(a) and (c)). The ET(30) increases upon increasing the number of ethanolic (–CH2–CH2–OH) moieties in the HBD component. The trend of CO2 against α and β indicates that higher CO2 uptake can be achieved in DESs by complexing less acidic and strongly basic moieties (Fig. 4(b)). Contrary to [ChCl]- and [DETA.Cl]-classes of DESs, [TBAB]-based DESs possess lower ET(30), smaller α but higher β. The [ChCl]-based DESs have higher ET(30) but comparable α and β values than the [TBAB]-class of DESs. Though the trend of ET(30), α and β against the CO2 uptake is similar in [ChCl]- and [TBAB]-based DESs, higher CO2 wt% is noted in [ChCl]-based DESs than in the [TBAB]-class of DESs with identical HBDs. However, none of these polarity parameters correlate with the CO2 w/w% in all DESs.
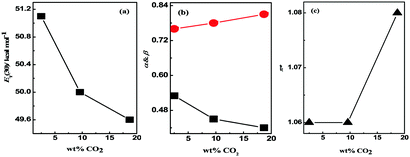 | ||
Fig. 4 A correlation between the CO2 uptake (wt%) and (a) ET(30), (b) α (■) and β ( ) and (c) π* (▲) in [TBAB]-based DESs. ) and (c) π* (▲) in [TBAB]-based DESs. | ||
A temperature-promoted CO2 capture study in ethylenediamine (EDA)-based DESs showed that rather than the hydrogen bond donor basicity (β), comparable α and β values correlate well with the CO2 w/w%.43 The comparable α and β cause “synergism” in the DES and result in higher CO2 w/w%. Synergistic interaction is noted as a thermodynamically controlled phenomenon and takes place under the positive set of enthalpy (ΔH°) and entropy (ΔS°) changes.43 In an ideal case, for maximum synergistic interaction |α–β| = 0. In a previous study, a near-zero |α–β| ≈ 0 is linked with the high CO2 uptake in DESs.43 The synergistic action is widely acknowledged in catalysis and polarity measurements of binary systems.43,44
Synergistic interaction was noted operative in the DESs employed for CO2 capture. [ChCl][EA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 showed higher CO2 uptake (29.2 wt%) than [TBAB][EA] = 1
6 showed higher CO2 uptake (29.2 wt%) than [TBAB][EA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (19.7 wt%) because of the equivalent α (0.64) and β (0.68) values in the former than the latter. Likewise, [ChCl][DEA] = 1
3 (19.7 wt%) because of the equivalent α (0.64) and β (0.68) values in the former than the latter. Likewise, [ChCl][DEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 has similar α and β and hence possesses higher CO2 uptake (19.6 wt%) than [TBAB][DEA] = 1
12 has similar α and β and hence possesses higher CO2 uptake (19.6 wt%) than [TBAB][DEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (9.6 wt%) for which β ≫ α. Analogously, higher CO2 uptake in [ChCl][TEA] = 1
2 (9.6 wt%) for which β ≫ α. Analogously, higher CO2 uptake in [ChCl][TEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in comparison with [TBAB][TEA] = 1
3 in comparison with [TBAB][TEA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 can be ascribed to the nearly lower |α–β| values.
2 can be ascribed to the nearly lower |α–β| values.
In the [DETA.Cl]-class of DESs, the strongest synergistic interaction prevailed in [DETA.Cl][EDA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and resulted in 32.2 wt% CO2 owing to the comparable but higher α and β values. [DETA.Cl][AP] = 1
4 and resulted in 32.2 wt% CO2 owing to the comparable but higher α and β values. [DETA.Cl][AP] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and [DETA.Cl][TEPA] = 1
4 and [DETA.Cl][TEPA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 have higher |α–β| and hence 18.3 wt% and 9.9 wt% of CO2 were obtained, respectively. The greater |α–β| causes an energy difference between the donor and acceptor moieties and disfavor high CO2 capture, whereas, the comparable α and β values bring the donor and acceptor together for optimum CO2 w/w%. Fig. 5 exhibits the dependence of CO2 wt% on |α–β| in [ChCl]- and [DETA.Cl]-based DESs.
4 have higher |α–β| and hence 18.3 wt% and 9.9 wt% of CO2 were obtained, respectively. The greater |α–β| causes an energy difference between the donor and acceptor moieties and disfavor high CO2 capture, whereas, the comparable α and β values bring the donor and acceptor together for optimum CO2 w/w%. Fig. 5 exhibits the dependence of CO2 wt% on |α–β| in [ChCl]- and [DETA.Cl]-based DESs.
The above discussion delineates the importance of synergistic interaction in high CO2 capture in DESs. The synergistic interaction becomes important in cases where a direct correlation of basicity or acidity with the observation is not feasible. The CO2 uptake analysis in [TBAB]-, [ChCl]- and [DETA.Cl]-based DESs represents one such case, where CO2 uptake resulting in the studied DESs can be explained based on the |α–β| values.
In conclusion, we have discussed the importance of the synergistic interaction upon CO2 capture in various classes of DESs. The above investigation demonstrates that neither the high Hammett basicity H− nor pKa results in high CO2 capture as noted in the case of [DETA.Cl][TEPA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (H− = 5.59 and β = 1.28). Alternatively, comparable α and β values in a DES establish synergistic interactions and favour high CO2 uptake. We believe that this study will motivate researchers to explore other important factors such as multiple-site- and intermolecular-interactions which will help in developing the understanding of acidic gas capture and conversion to useful products.
4 (H− = 5.59 and β = 1.28). Alternatively, comparable α and β values in a DES establish synergistic interactions and favour high CO2 uptake. We believe that this study will motivate researchers to explore other important factors such as multiple-site- and intermolecular-interactions which will help in developing the understanding of acidic gas capture and conversion to useful products.
This work was part of the activities of the Technical Chemistry, Department of Chemistry, Chemical–Biological Centre, Umeå University, Sweden, as well as the Johan Gadolin Process Chemistry Centre at Åbo Akademi University in Finland. The Bio4Energy programme, Kempe Foundations and Wallenberg Wood Science Center are gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.References
- B. Metz, O. Davidson, H. de Coninck, M. Loos and L. Meyer, Cambridge University Press, 2005, http://www.ipcc.ch/publications_and_data_reports.html#1, accessed 9.07.2010.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- F. Wang, J. Zhao, H. Miao, J. Zhao, H. Zhang, J. Yuan and J. Yan, Appl. Energy, 2018, 230, 734–749 CrossRef CAS.
- G. Astarita, D. W. Savage and A. Bisio, Gas treating with chemical solvents, John Wiley & Sons, New York, 1983 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 399, 28–29 CrossRef.
- K. R. Seddon, Nature, 2003, 2, 363–365 CrossRef CAS PubMed.
- Y. H. Chen, S. J. Zhang, X. L. Yuan, Y. Q. Zhang, X. P. Zhang, W. B. Dai and R. Mori, Thermochim. Acta, 2006, 441, 42–44 CrossRef CAS.
- X. L. Yuan, S. J. Zhang, J. Liu and X. M. Lu, Fluid Phase Equilib., 2007, 257, 195–200 CrossRef CAS.
- D. Wappel, G. Gronald, R. Kalb and J. Draxler, Int. J. Greenhouse Gas Control, 2010, 4, 486–494 CrossRef CAS.
- M. Hasib-ur-Rahman, M. Siaj and F. Larachi, Chem. Eng. Process., 2010, 49, 313–322 CrossRef CAS.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed.
- B. E. Gurkan, J. C. de la Fuente, E. M. Mindrup, L. E. Ficke, B. F. Goodrich, E. A. Price, W. F. Schneider and J. F. Brennecke, J. Am. Chem. Soc., 2010, 132, 2116–2117 CrossRef CAS PubMed.
- B. F. Goodrich, J. C. de la Fuente, B. E. Gurkan, D. J. Zadigian, E. A. Price, Y. Huang and J. F. Brennecke, Ind. Eng. Chem. Res., 2011, 50, 111–118 CrossRef CAS.
- Y. Zhang, S. Zhang, X. Lu, Q. Zhou, W. Fan and X. Zhang, Chem. – Eur. J., 2009, 15, 3003–3011 CrossRef CAS PubMed.
- C. Wang, X. Luo, H. Luo, D.-E. Jiang, H. Li and S. Dai, Angew. Chem., Int. Ed., 2011, 50, 4918–4922 CrossRef CAS PubMed.
- H. Tang and C. Wu, ChemSusChem, 2013, 6, 1050–1056 CrossRef CAS PubMed.
- B. Gurkan, B. F. Goodrich, E. M. Mindrup, L. E. Ficke, M. Massel, S. Seo, T. P. Senftle, H. Wu, M. F. Glaser, J. K. Shah, E. J. Maginn, J. F. Brennecke and W. F. J. Schneider, J. Phys. Chem. Lett., 2010, 1, 3494–3499 CrossRef CAS.
- T. Oncsik, R. Vijayaraghavan and D. R. MacFarlane, Chem. Commun., 2018, 54, 2106–2109 RSC.
- Y. Wu, Y. Zhao, R. Li, B. Yu, Y. Chen, X. Liu, C. Wu, X. Luo and Z. Liu, ACS Catal., 2017, 7, 6251–6255 CrossRef CAS.
- C. Wang, H. Luo, D. Jiang, H. Li and S. Dai, Angew. Chem., Int. Ed., 2010, 49, 5978–5981 CrossRef CAS PubMed.
- L. M. Galan Sanchez, G. W. Meindersma and A. B. de Haan, Chem. Eng. Res. Des., 2007, 85, 31–39 CrossRef.
- P. J. Carvalho, K. A. Kurnia and J. A. P. Coutinho, Phys. Chem. Chem. Phys., 2016, 18, 14757–14771 RSC.
- X. M. Liu, G. H. Zhou, S. J. Zhang and X. Q. Yao, Fluid Phase Equilib., 2009, 284, 44–49 CrossRef CAS.
- J. M. Zhang, S. J. Zhang, K. Dong, Y. Q. Zhang, Y. Q. Shen and X. M. Lv, Chem. – Eur. J., 2006, 12, 4021–4026 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- A. Abo-Hamad, M. Hayyan, M. A. H. AlSaadi and M. A. Hashim, Chem. Eng. J., 2015, 273, 551–567 CrossRef CAS.
- B. Tang, H. Zhang and K. H. Row, J. Sep. Sci., 2015, 38, 1053–1064 CrossRef CAS.
- J. García-Álvarez, Eur. J. Inorg. Chem., 2015, 5147–5157 CrossRef.
- C. Vidal, J. García-Álvarez, A. Hernán-Gómez, A. R. Kennedy and E. Hevia, Angew. Chem., Int. Ed., 2016, 55, 16145–16148 CrossRef CAS.
- Y. Zhang, X. Ji and X. Lu, Renewable Sustainable Energy Rev., 2018, 97, 436–455 CrossRef CAS.
- L. Cao, J. Huang, X. Zhang, S. Zhang, J. Gao and S. Zeng, Phys. Chem. Chem. Phys., 2015, 17, 27306–27316 RSC.
- T. J. Trivedi, J. H. Lee, H. J. Lee, Y. K. Jeong and J. W. Choi, Green Chem., 2016, 18, 2834–2842 RSC.
- K. Zhang, Y. Hou, Y. Wang, K. Wang, S. Ren and W. Wu, Energy Fuels, 2018, 32, 7727–7733 CrossRef CAS.
- P. J. Carvalho and J. A. P. Coutinho, Energy Environ. Sci., 2011, 4, 4614–4619 RSC.
- G. Cui, M. Lva and D. Yang, Chem. Commun., 2019, 55, 1426–1429 RSC.
- B. Jiang, J. Ma, N. Yang, Z. Huang, N. Zhang, X. Tantai, Y. Sun and L. Zhang, Energy Fuels, 2019, 33(8), 7569–7577 CrossRef CAS.
- S. Zeng, X. Zhang, L. Bai, X. Zhang, H. Wang, J. Wang, D. Bao, M. Li, X. Liu and S. Zhang, Chem. Rev., 2017, 117(14), 9625–9673 CrossRef CAS PubMed.
- F.-F. Chen, K. Huang, Y. Zhou, Z.-Q. Tian, X. Zhu, D.-J. Tao, D.-E. Jiang and S. Dai, Angew. Chem., Int. Ed., 2016, 55, 7166–7170 CrossRef CAS PubMed.
- S. K. Shukla and J.-P. Mikkola, Phys. Chem. Chem. Phys., 2018, 20, 24591–24601 RSC.
- S. K. Shukla and J.-P. Mikkola, Chem. Commun., 2019, 55, 3939–3942 RSC.
- L. Hongliang, W. Liangbing, D. Yizhou, P. Zhengtian, L. Zhuhan, C. Yawei, W. Menglin, Z. Xusheng, Z. Junfa, Z. Wenhua, S. Rui, M. Chao and Z. Jie, Nat. Nanotechnol., 2018, 13, 11–417 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of DESs, experimental methods etc. See DOI: 10.1039/c9cp06017k |
| This journal is © the Owner Societies 2020 |


