Unusual behaviour of the spin–spin coupling constant 1JCH upon formation of CH⋯X hydrogen bond†
Abstract
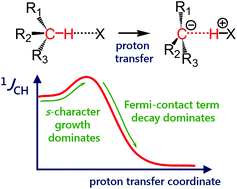
One-bond coupling constants 1JXY are usually used as a measure of the corresponding X⋯Y interatomic distances. However, the physical nature of this correlation is not well understood and, in some cases, a counterintuitive behaviour of 1JXY upon hydrogen bonded complex formation has been reported. In this work, the behavior of 1JCH upon formation and strengthening of complexes with CH⋯X hydrogen bonds and upon a proton transfer process is investigated by means of 1H NMR spectroscopy and quantum chemical calculations. 1H NMR spectra of 1,1-dinitroethane solution at room temperature in various solvents (carbon tetrachloride, chloroform, dichloromethane, acetone, dimethylformamide and dimethyl sulfoxide) illustrate the increase of 1JCH by several Hz upon an increase of the complex strength. Computational results (MP2/aug-cc-pVDZ) reproduce this observation and allow one to conclude that the increase of 1JCH is mainly caused by the change of the carbon hybridization (an increase of s-character), rather than by the change in interatomic distance rCH. The behavior of 1JCH was also examined computationally for a wide range of CH⋯X hydrogen bond energies and geometries. For this purpose, quantum-chemical modeling of the partial proton transfer process for complexes formed by 1,1-dinitroethane and trinitromethane as hydrogen bond donors with acetone, pyridine and fluoride anion as hydrogen bond acceptors was performed. The obtained results have confirmed the above-mentioned idea – for rather weak complexes, the dominant impact on the change of 1JCH magnitude is the increase of the s-character of carbon atom hybridization, while for complexes with a significantly transferred proton, the exponential decrease of the Fermi-contact term dominates.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...