Time-resolved FTIR study on the structural switching of human galectin-1 by light-induced disulfide bond formation†
Abstract
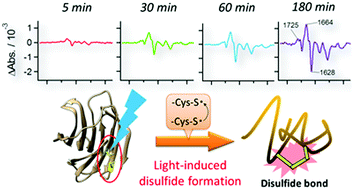
Disulfide bonds play a fundamental role in controlling the tertiary structure of proteins; the formation or cleavage of some disulfide bonds can switch the structures and/or functions of proteins. Human galectin-1 (hGal-1), which is a lectin protein, exemplifies how both structure and function are changed by disulfide bonds; the structure and sugar-binding ability of hGal-1 are altered by the formation and cleavage of its three intra-molecular disulfide bonds. In the present study, the dynamics of the structural change of hGal-1 by the formation of disulfide bonds were investigated by time-resolved FTIR spectroscopy combined with a modification in which its thiol groups (–SH) were replaced with S-nitrosylated groups (SNO). Photodissociation of NO from SNO in reduced hGal-1 induced disulfide bond formation and transformed it into the oxidised form. The structural change to the oxidised form involved three distinct kinetics with fast (<300 s), middle (∼600 s), and slow (∼6400 s) lifetimes. In an examination of hGal-1 in the lactose-bound form, structural changes owing to the release of substrate lactose were also observed upon disulfide bond formation. The present method using the photodissociation of NO is useful for monitoring the dynamics of structural changes following disulfide formation.


 Please wait while we load your content...
Please wait while we load your content...