Interactions of a multifunctional di-triazole derivative with Alzheimer's Aβ42 monomer and Aβ42 protofibril: a systematic molecular dynamics study†
Abstract
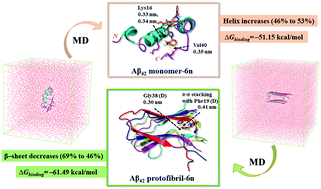
Amyloid aggregation modulators offer a promising treatment strategy for Alzheimer's disease (AD). We have recently reported a novel di-triazole based compound 6n as a multi-target-directed ligand (MTDL) against AD. 6n effectively inhibits Aβ42 aggregation, metal-induced Aβ42 aggregation, reactive oxygen species (ROS) generation, and rescues SH-SY5Y cells from Aβ42 induced neurotoxicity. However, the underlying inhibitory mechanism remains uncovered. In this regard, molecular dynamics (MD) simulations were performed to understand the effect of 6n on the structure and stability of monomeric Aβ42 and a pentameric protofibril structure of Aβ42. Compound 6n binds preferably to the central hydrophobic core (CHC) and C-terminal regions of the Aβ42 monomer as well as the protofibril structure. The secondary structure analysis suggests that 6n prevents the aggregation of the Aβ42 monomer and disaggregates Aβ42 protofibrils by sustaining the helical content in the Aβ42 monomer and converting the β-sheet into random coil conformation in the Aβ42 protofibril structure. A significant decrease in the average number of hydrogen bonds, binding affinity, and residue–residue contacts between chains D–E of the Aβ42 protofibril in the presence of 6n indicates destabilization of the Aβ42 protofibril structure. The MM-PBSA (molecular mechanics Poisson–Boltzmann surface area) analysis highlighted favourable binding free energy (ΔGbinding) for the Aβ42 monomer–6n and Aβ42 protofibril–6n complex. Overall, MD results highlighted that 6n stabilizes the native α-helix conformation of the Aβ42 monomer and induces a sizable destabilization in the Aβ42 protofibril structure. This work provides theoretical insights into the inhibitory mechanism of 6n against amyloid aggregation and will be beneficial as a molecular guide for structure-based drug design against AD.


 Please wait while we load your content...
Please wait while we load your content...