Open Access Article
Open Access ArticlePressure-induced phase separation of miscible liquids: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 1 n-pentane/iso-pentane†
1 n-pentane/iso-pentane†
X.
Liu
 * and
C. R.
Pulham
* and
C. R.
Pulham

EaStCHEM School of Chemistry and Centre for Science at Extreme Conditions, University of Edinburgh, Joseph Black Building, Edinburgh EH9 3FJ, Scotland, UK. E-mail: Xiaojiao.Liu@ed.ac.uk
First published on 16th November 2020
Abstract
We report the unexpected pressure-induced and time-dependent nucleation and crystallisation of n-pentane from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of n-pentane and iso-pentane. The crystal structure of n-pentane at 3.3 GPa is reported; the 19.6% decrease in volume up to 3.3 GPa is caused by the significant reduction of voids within the structure. The study has wider implications for the separation of mixtures of organic compounds that have similar melting and boiling points, as well as the use of a 1
1 mixture of n-pentane and iso-pentane. The crystal structure of n-pentane at 3.3 GPa is reported; the 19.6% decrease in volume up to 3.3 GPa is caused by the significant reduction of voids within the structure. The study has wider implications for the separation of mixtures of organic compounds that have similar melting and boiling points, as well as the use of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of n-pentane and iso-pentane as a pressure-transmitting medium in high-pressure diffraction experiments.
1 mixture of n-pentane and iso-pentane as a pressure-transmitting medium in high-pressure diffraction experiments.
Introduction
The separation of miscible liquids with similar boiling points represents a significant challenge for both industrial and academic fields. For example, the petrochemical industry invests heavily in the development of efficient techniques for the separation and refinement of crude oil in order to manufacture high-quality commercial fuels and petrochemicals. Current methods typically involve the separation of crude oil using distillation into different fractions with specific boiling ranges and distributions of components with different carbon number.1 Such processes consume significant amounts of energy with consequential emission of CO2.2,3 Nevertheless, the separation of mixtures of aliphatic or aromatic hydrocarbons that contain similar numbers of carbon atoms and which have similar melting and boiling points remains very challenging.Several experimental and computational studies have been conducted to explore the potential of zeolites and metal–organic frameworks for the separation of isomers of hydrocarbons such as hexane and xylene.4,5 However, this remains a challenge due to the low efficiency of separation, as the diffusivities of molecules within zeolites are typically very low.4 A technique that can be used to separate mixtures that have very different freezing points is selective crystallisation. For example, ethanol/water mixtures can be partially separated by low-temperature crystallisation in which water ice preferentially crystallises to leave a solution that is more concentrated in ethanol.6
In this work we demonstrate how pressure-induced crystallisation can be used to separate two compounds with very similar melting and boiling points that are completely miscible under normal conditions of temperature and pressure.
Iso-pentane (2-methylbutane) and n-pentane are structural isomers with molecular formula C5H12 (Fig. 1), and under normal conditions of temperature and pressure they are freely miscible at all compositions. The normal boiling points of the two compounds are 300.8 K and 309.1 K, respectively, and so separation of the two components by distillation is extremely difficult. At atmospheric pressure, pure iso-pentane solidifies into an amorphous, glassy phase at 113.4 K, whereas n-pentane freezes at 143.4 K to give a crystalline phase.7,8 Using laser heating, a single crystal was grown in a capillary at 115 K and single-crystal X-ray diffraction data were recorded at 90 K.9 Under these conditions n-pentane crystallises in the orthorhombic crystal system with space group Pbcn (Table 1).9
| a (Å) | b (Å) | c (Å) | V (Å)3 | ρ (kg m−3) | |
|---|---|---|---|---|---|
| High pressure (3.3 GPa & 298 K) | 3.7996(7) | 8.2477(13) | 14.205(4) | 445.16(17) | 1076.1 |
| Ambient pressure (90 K)9 | 4.1357(8) | 9.025(3) | 14.816(5) | 553.00(12) | 866.6 |
Increasing pressure can often induce similar crystallisation behaviour as cooling, although initial studies by Bridgman noted that n-pentane did not solidify even up to pressures of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 N cm−2 (2.94 GPa).10 Gelles calculated its pressure-induced freezing point at 1.755 ± 0.067 GPa (296.3 ± 0.3 K), using the Clapeyron equation.11 This result is in a good agreement with experimental studies by Reeves et al. that extrapolated the freezing pressure of n-pentane to be 1.5 GPa at 298 K.12 The same study also extrapolated the freezing pressure of iso-pentane to be 2.1 GPa at 298 K. Spectroscopic studies have shown that n-pentane crystallises at ca. 2.5 GPa and 295 K,13,14 and that there is some evidence for a new high-pressure phase of n-pentane in the pressure range of 2.84 to 4.77 GPa.14 Based on Raman spectroscopic studies, Williams suggested that the high-pressure crystalline phase of n-pentane crystallises in the orthorhombic crystal system.8,15 However, the crystal structure of n-pentane at high-pressure conditions has not yet been reported.
000 N cm−2 (2.94 GPa).10 Gelles calculated its pressure-induced freezing point at 1.755 ± 0.067 GPa (296.3 ± 0.3 K), using the Clapeyron equation.11 This result is in a good agreement with experimental studies by Reeves et al. that extrapolated the freezing pressure of n-pentane to be 1.5 GPa at 298 K.12 The same study also extrapolated the freezing pressure of iso-pentane to be 2.1 GPa at 298 K. Spectroscopic studies have shown that n-pentane crystallises at ca. 2.5 GPa and 295 K,13,14 and that there is some evidence for a new high-pressure phase of n-pentane in the pressure range of 2.84 to 4.77 GPa.14 Based on Raman spectroscopic studies, Williams suggested that the high-pressure crystalline phase of n-pentane crystallises in the orthorhombic crystal system.8,15 However, the crystal structure of n-pentane at high-pressure conditions has not yet been reported.
It is well known that miscible mixtures of components introduce an additional contribution to the entropy of the system and reduce the freezing point of the mixture. In a similar way, the freezing pressure of a liquid can be increased by mixing multiple components. For example, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (by volume) of n-pentane and iso-pentane is frequently used as a pressure-transmitting medium in high-pressure experiments in order to maintain a hydrostatic environment for the sample. Measurements by Barnett and Bosco show a continuous increase in the viscosity up to 5.4 GPa where it reaches 108 cP, corresponding approximately to “the viscosity of window putty”.16 More recent investigations on the 1
1 mixture (by volume) of n-pentane and iso-pentane is frequently used as a pressure-transmitting medium in high-pressure experiments in order to maintain a hydrostatic environment for the sample. Measurements by Barnett and Bosco show a continuous increase in the viscosity up to 5.4 GPa where it reaches 108 cP, corresponding approximately to “the viscosity of window putty”.16 More recent investigations on the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture using a strain-gauge technique indicate a freezing pressure of 5.6 GPa at ambient temperature.17 The freezing pressure of this mixture has been reported to be as high as ca. 7.4 GPa at room temperature as measured by the ruby-fluorescence method.18 Klotz et al. reported that the 1
1 mixture using a strain-gauge technique indicate a freezing pressure of 5.6 GPa at ambient temperature.17 The freezing pressure of this mixture has been reported to be as high as ca. 7.4 GPa at room temperature as measured by the ruby-fluorescence method.18 Klotz et al. reported that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture solidifies in the temperature range of 100–120 K at 10−4 GPa and between 138–148 K at 0.7 GPa to form an amorphous glass.19 An additional advantage of using this mixture as a pressure-transmitting medium is that on pressure-induced freezing, a homogenous amorphous phase is formed,10 which means that no additional Bragg peaks are introduced into the patterns obtained from diffraction experiments, nor phonon vibrational bands in spectroscopic measurements.18,20 Moreover, both n-pentane and iso-pentane are relatively unreactive and so do not interact chemically with samples under investigation.
1 mixture solidifies in the temperature range of 100–120 K at 10−4 GPa and between 138–148 K at 0.7 GPa to form an amorphous glass.19 An additional advantage of using this mixture as a pressure-transmitting medium is that on pressure-induced freezing, a homogenous amorphous phase is formed,10 which means that no additional Bragg peaks are introduced into the patterns obtained from diffraction experiments, nor phonon vibrational bands in spectroscopic measurements.18,20 Moreover, both n-pentane and iso-pentane are relatively unreactive and so do not interact chemically with samples under investigation.
Experimental
Anhydrous n-pentane (purity = 99.8%) and iso-pentane (purity ≥ 99%) were purchased from Fisher Scientific UK Ltd. For all experiments, the mixture of n-pentane/iso-pentane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was freshly mixed and loaded cold into a Merrill–Bassett diamond-anvil cell equipped with 600 μm culet diamonds and a steel gasket with a 300 μm diameter hole, with ruby spheres as pressure calibrants. The mixture was loaded inside a laboratory freezer operating at ca. 256 K, and under associated low-humidity conditions. An assembled DAC was loaded with a crystal of Bipy:NTO and ruby spheres and placed in the freezer, together with the 1
1) was freshly mixed and loaded cold into a Merrill–Bassett diamond-anvil cell equipped with 600 μm culet diamonds and a steel gasket with a 300 μm diameter hole, with ruby spheres as pressure calibrants. The mixture was loaded inside a laboratory freezer operating at ca. 256 K, and under associated low-humidity conditions. An assembled DAC was loaded with a crystal of Bipy:NTO and ruby spheres and placed in the freezer, together with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture contained in a syringe. After cooling for 30 min the DAC was quickly sealed and compressed gently in order to prevent the evaporation of the pentanes. HP-XRSD data were collected at 298 K using a Mo-Kα radiation source on a Bruker D8 Venture in the School of Chemistry at the University of Edinburgh. Bruker APEX III software package was used for data processing and reduction, Indexing was performed using the “Cell Now” program.21 Crystal structure solution and refinements were performed using the Olex 2 program.22
1 mixture contained in a syringe. After cooling for 30 min the DAC was quickly sealed and compressed gently in order to prevent the evaporation of the pentanes. HP-XRSD data were collected at 298 K using a Mo-Kα radiation source on a Bruker D8 Venture in the School of Chemistry at the University of Edinburgh. Bruker APEX III software package was used for data processing and reduction, Indexing was performed using the “Cell Now” program.21 Crystal structure solution and refinements were performed using the Olex 2 program.22
Results and discussion
In this paper we report the unexpected selective crystallisation of n-pentane (phase separation) from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of n-pentane and iso-pentane under high-pressure conditions whilst using this 1
1 mixture of n-pentane and iso-pentane under high-pressure conditions whilst using this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture as a pressure-transmitting medium in a diamond-anvil cell (DAC). The initial experiment involved step-wise compression of a single crystal of a 1
1 mixture as a pressure-transmitting medium in a diamond-anvil cell (DAC). The initial experiment involved step-wise compression of a single crystal of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal containing 4,4′-bipyridine and nitrotriazolone (Bipy:NTO), together with several ruby spheres which act as a pressure calibrant. The yellow crystal was initially compressed to ca. 0.12 GPa and then increased in steps of approximately 0.5 GPa, with single-crystal X-ray diffraction measurements recorded at each pressure point. The DAC was allowed to equilibrate for ca. 20 h at each pressure point, in order to stabilise the pressure in the sample chamber. On account of the weakly diffracting crystal, data-collection times of ∼38 hours were required (further details of Bipy:NTO will be the subject of a future publication). Diffraction data were collected for the sample crystal up to 2.8 GPa, but after ∼18 hours into the data collection at this pressure, multiple colourless crystallites were observed in the chamber of the DAC (Fig. 2).
1 co-crystal containing 4,4′-bipyridine and nitrotriazolone (Bipy:NTO), together with several ruby spheres which act as a pressure calibrant. The yellow crystal was initially compressed to ca. 0.12 GPa and then increased in steps of approximately 0.5 GPa, with single-crystal X-ray diffraction measurements recorded at each pressure point. The DAC was allowed to equilibrate for ca. 20 h at each pressure point, in order to stabilise the pressure in the sample chamber. On account of the weakly diffracting crystal, data-collection times of ∼38 hours were required (further details of Bipy:NTO will be the subject of a future publication). Diffraction data were collected for the sample crystal up to 2.8 GPa, but after ∼18 hours into the data collection at this pressure, multiple colourless crystallites were observed in the chamber of the DAC (Fig. 2).
Weak peaks in the diffraction patterns were also observed arising from these crystallites (see ESI† for details). On raising the pressure to 3.3 GPa, the co-crystal accidentally lost crystallinity and became amorphous. In the absence of Bragg peaks from the sample, it was then possible to index the diffraction peaks from several of the colourless crystallites to obtain plausible orthorhombic unit-cell parameters with space group Pbcn (Table 1).
The structure (CCDC No. 2026696) was then solved and refined to give an R-factor of 3.67%. Further crystallographic parameters are provided in the ESI.† The structure is consistent with that previously reported for the low-temperature crystal structure of n-pentane, taking into account the effects of pressure on the lattice parameters.9 Compression is clearly anisotropic over this pressure range: the b-axis and a-axis decrease by 8.66% and 8.16%, respectively, whereas the c-axis decreases by only 4.26%. This can be explained by the observation that the molecules of n-pentane align along the c-axis (Fig. 3a), and so the length of the c-axis is determined by the C–C covalent bonds within the hydrocarbon chains. These are relatively incompressible bonds, and so would not be expected to be strongly affected by pressure. Overall, there is a 19.6% decrease in volume over this pressure range, which is typical for molecular compounds where relatively weak van der Waals interactions dominate. This is demonstrated by the significant reduction of voids in the structure at 3.3 GPa, compared with the ambient-pressure structure (Fig. 3b and c).
Careful analysis of the diffraction patterns showed that no other crystalline phases were present, thus indicating that the iso-pentane component remains in the liquid phase despite being above its reported extrapolated freezing pressure of 2.1 GPa.12 Confirmation that the iso-pentane remained liquid is provided by the absence of any significant broadening of the ruby fluorescence signal (ESI†). Presumably this is because some n-pentane remains dissolved in the iso-pentane, thereby raising the freezing pressure of the mixture. No evidence was observed for the potential pressure-induced phase transition of n-pentane in the pressure range of 2.84 to 4.77 GPa reported by Qiao et al.,14 but this may reflect the rather different crystallisation conditions between the two studies.
The reason for the unexpected nucleation and subsequent crystallisation of n-pentane was initially not clear. One possibility is that the sample of the co-crystal Bipy:NTO was responsible for inducing nucleation, but this was ruled out by repeating the compression of a fresh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture in the absence of Bipy:NTO. Fig. 4a shows the optical image of the sample on first compressing to 2.6 GPa – no crystallisation was observed. However, on standing at this pressure and at 298 K for 46 hours, a few small crystals of n-pentane appeared in the DAC (Fig. 4b). This behaviour was very reproducible and crystallisation was observed on multiple occasions under similar experimental conditions (see ESI†). It became clear that a key factor required for successful nucleation is time – in all cases an induction period of multiple hours at ambient temperature was required in order to initiate nucleation and subsequent crystal growth. Our explanation for the observed behaviour is therefore as follows. At elevated pressures, it becomes thermodynamically favourable for n-pentane to crystallise from the mixture as a pure solid, presumably driven by the large decrease in molar volume of the crystalline solid compared with that of the fluid mixture, thereby substantially reducing the free energy of solid n-pentane. However, the high viscosity of the fluid means that diffusion-controlled nucleation and subsequent crystal growth are both slow. It is interesting to note that with relatively large pressure steps and shorter equilibration times, the mixture did not phase separate and instead froze to give a homogeneous glassy phase at the hydrostatic limit of the binary mixture (7.4 GPa). This is because the viscosity of the mixture increases very rapidly with increasing pressure and becomes so high that molecular diffusion is drastically suppressed and nucleation cannot occur.
1 mixture in the absence of Bipy:NTO. Fig. 4a shows the optical image of the sample on first compressing to 2.6 GPa – no crystallisation was observed. However, on standing at this pressure and at 298 K for 46 hours, a few small crystals of n-pentane appeared in the DAC (Fig. 4b). This behaviour was very reproducible and crystallisation was observed on multiple occasions under similar experimental conditions (see ESI†). It became clear that a key factor required for successful nucleation is time – in all cases an induction period of multiple hours at ambient temperature was required in order to initiate nucleation and subsequent crystal growth. Our explanation for the observed behaviour is therefore as follows. At elevated pressures, it becomes thermodynamically favourable for n-pentane to crystallise from the mixture as a pure solid, presumably driven by the large decrease in molar volume of the crystalline solid compared with that of the fluid mixture, thereby substantially reducing the free energy of solid n-pentane. However, the high viscosity of the fluid means that diffusion-controlled nucleation and subsequent crystal growth are both slow. It is interesting to note that with relatively large pressure steps and shorter equilibration times, the mixture did not phase separate and instead froze to give a homogeneous glassy phase at the hydrostatic limit of the binary mixture (7.4 GPa). This is because the viscosity of the mixture increases very rapidly with increasing pressure and becomes so high that molecular diffusion is drastically suppressed and nucleation cannot occur.
Above 2.5 GPa and before crystallisation, one can envisage the fluid as a metastable system comprising a solution of the solute (n-pentane) dissolved in a solvent (iso-pentane), such that the concentration of the solution lies within its metastable zone width (MSZW), i.e. the region of maximum allowable supersaturation. Over time and provided that the viscosity is not too high, the solute is able to nucleate and crystallise from the solution.
A common method to encourage nucleation is to decrease the temperature of a solution in order to increase the degree of supersaturation. This was demonstrated by initially compressing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
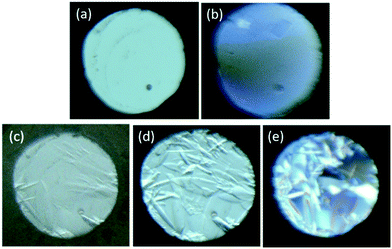
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of n-pentane and iso-pentane to 2.3 GPa, at which point no crystallisation was observed. On cooling the DAC to 256 K (in the freezer) and holding at this temperature for 16 hours, several crystallites were observed to grow (Fig. 4c). Further brief (20 min) cooling to 195 K (in a dry-ice bath), followed by warming to ambient temperature (Fig. 4d) resulted in the formation of many more crystallites, again suggesting that cooling reduced the solubility of the n-pentane in the high-pressure fluid. After a further 16 hours at ambient temperature the crystals appeared larger (Fig. 4e), perhaps suggesting some degree of Ostwald ripening.
1 mixture of n-pentane and iso-pentane to 2.3 GPa, at which point no crystallisation was observed. On cooling the DAC to 256 K (in the freezer) and holding at this temperature for 16 hours, several crystallites were observed to grow (Fig. 4c). Further brief (20 min) cooling to 195 K (in a dry-ice bath), followed by warming to ambient temperature (Fig. 4d) resulted in the formation of many more crystallites, again suggesting that cooling reduced the solubility of the n-pentane in the high-pressure fluid. After a further 16 hours at ambient temperature the crystals appeared larger (Fig. 4e), perhaps suggesting some degree of Ostwald ripening.
Conclusions
We have observed the unexpected pressure-induced crystallisation of n-pentane from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of n-pentane and iso-pentane. This has been interpreted on the basis that at elevated pressures, it becomes thermodynamically favourable for n-pentane to crystallise from the mixture as a pure solid, because of the large decrease in molar volume of the crystalline solid compared with that of the fluid mixture, thereby substantially reducing the free energy of solid n-pentane. However, the high viscosity of the fluid means that diffusion-controlled nucleation and subsequent crystal growth are highly time-dependent. The crystal structure of n-pentane at 3.3 GPa has been determined and demonstrates anisotropic compression of the unit cell such that the c-axis is the least compressible. This can be rationalised the observation that the molecules of n-pentane align along the c-axis and C–C bonds are relatively incompressible. Overall, there is a 19.6% decrease in volume up to 3.3 GPa caused by the significant reduction of voids within the structure.
1 mixture of n-pentane and iso-pentane. This has been interpreted on the basis that at elevated pressures, it becomes thermodynamically favourable for n-pentane to crystallise from the mixture as a pure solid, because of the large decrease in molar volume of the crystalline solid compared with that of the fluid mixture, thereby substantially reducing the free energy of solid n-pentane. However, the high viscosity of the fluid means that diffusion-controlled nucleation and subsequent crystal growth are highly time-dependent. The crystal structure of n-pentane at 3.3 GPa has been determined and demonstrates anisotropic compression of the unit cell such that the c-axis is the least compressible. This can be rationalised the observation that the molecules of n-pentane align along the c-axis and C–C bonds are relatively incompressible. Overall, there is a 19.6% decrease in volume up to 3.3 GPa caused by the significant reduction of voids within the structure.
Given the wider challenges associated with the separation of mixtures of organic compounds that have similar melting and boiling points, this study points to the potential use of pressure-induced crystallisation (especially when combined with temperature) as a means to achieve this goal. Whilst the pressures used in the current study are not readily achievable on an industrial (batch) scale, one could envisage smaller scale pressure vessels operating in semi-continuous mode. Moreover, the pentanes used in this study have very normal low melting points and hence unusually high freezing pressures at ambient temperature. By contrast, the freezing pressures of many liquids lie well within easily accessible pressure ranges – for example the freezing pressure of acetic acid at 298 K is only 0.2 GPa.23
Finally, because 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of n-pentane and iso-pentane (and their perdeuterated isotopomers) are often used as pressure-transmitting media in high-pressure X-ray and neutron powder diffraction experiments, one significant implication for the unexpected crystallisation of n-pentane is that such experiments may be prone to the appearance of unexpected diffraction peaks associated with crystallisation of n-pentane. In Experiments that involve long data-collection times where samples are maintained at elevated pressures (in the range 2–4 GPa) for long periods of time might therefore be particularly prone to this, e.g. collection of high-pressure single crystal data using laboratory X-ray sources or high-pressure neutron powder diffraction experiments using a Paris–Edinburgh press.24
1 mixtures of n-pentane and iso-pentane (and their perdeuterated isotopomers) are often used as pressure-transmitting media in high-pressure X-ray and neutron powder diffraction experiments, one significant implication for the unexpected crystallisation of n-pentane is that such experiments may be prone to the appearance of unexpected diffraction peaks associated with crystallisation of n-pentane. In Experiments that involve long data-collection times where samples are maintained at elevated pressures (in the range 2–4 GPa) for long periods of time might therefore be particularly prone to this, e.g. collection of high-pressure single crystal data using laboratory X-ray sources or high-pressure neutron powder diffraction experiments using a Paris–Edinburgh press.24
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr. Gary Nichol for discussions about crystal structure solution. Dr. X. Liu thanks EPSRC for funding.Notes and references
- L. T. Ikelle and L. Amundsen, Introduction to Petroleum Seismology, 2005 Search PubMed.
- W. R. Paterson, Petroleum refining: technology and economics 3rd edn., Marcel Dekker Inc, New York, 3rd edn, 1994 Search PubMed.
- J. G. Speight, Environmental Analysis and Technology for the Refining Industry, 2006 Search PubMed.
- A. Luna-Triguero, P. Gómez-Álvarez and S. Calero, Phys. Chem. Chem. Phys., 2017, 19, 5037 RSC.
- Q. Shi, J. C. Gonçalves, A. F. P. Ferreira, M. G. Plaza and A. E. Rodrigues, Ind. Eng. Chem. Res., 2018, 57, 5568–5579 CrossRef CAS.
- G. Fix, Principles of Brewing Science: a study of serious brewing issues, 1999 Search PubMed.
- L. E. Reeves, G. J. Scott and S. E. Babb, J. Chem. Phys., 1964, 40, 3662–3666 CrossRef CAS.
- D. E. Williams, J. Chem. Phys., 1967, 47, 4680–4684 CrossRef CAS.
- R. Boese, H. Weiss and D. Blaeser, Angew. Chem., Int. Ed., 1999, 38, 988–992 CrossRef CAS.
- P. W. Bridgman, Proc. Am. Acad. Arts Sci., 1941, 77, 117 Search PubMed.
- S. H. Gelles, J. Chem. Phys., 1968, 48, 526–527 CrossRef CAS.
- L. E. Reeves, G. J. Scott and S. E. Babb, J. Chem. Phys., 1964, 40, 3662 CrossRef CAS.
- S. A. Lee, A. Anderson, S. M. Lindsay and R. C. Hanson, High Pressure Res., 1990, 3, 230–232 CrossRef.
- E. Qiao and H. Zheng, Appl. Spectrosc., 2005, 59, 650–653 CrossRef CAS.
- H. G. Olf and B. Fanconi, J. Chem. Phys., 1973, 59, 534–544 CrossRef CAS.
- J. D. Barnett and C. D. Bosco, J. Appl. Phys., 1969, 40, 3144–3150 CrossRef CAS.
- M. Nomura, T. Nishizaka, Y. Hirata, N. Nakagiri and H. Fujiwara, Jpn. J. Appl. Phys., 1982, 21, 936 CrossRef CAS.
- S. Klotz, J.-C. Chervin, P. Munsch and G. Le Marchand, J. Phys. D: Appl. Phys., 2009, 42, 75413 CrossRef.
- S. Klotz, J. Philippe and E. Cochard, J. Phys. D: Appl. Phys., 2006, 39, 1674–1677 CrossRef CAS.
- M. S. Torikachvili, S. K. Kim, E. Colombier, S. L. Bud'Ko and P. C. Canfield, Rev. Sci. Instrum., 2015, 86, 123904 CrossRef CAS.
- G. M. Sheldrick, Cell_Now, University of Göttingen, Germany, 2008 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- D. R. Allan and S. J. Clark, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 6328 CrossRef CAS.
- C. L. Bull, N. P. Funnell, M. G. Tucker, S. Hull, D. J. Francis and W. G. Marshall, High Pressure Res., 2016, 36, 493–511 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2026696. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ce01335h |
| This journal is © The Royal Society of Chemistry 2020 |