MOF-derived yolk–shell Ni/C architectures assembled with Ni@C core–shell nanoparticles for lightweight microwave absorbents†
Abstract
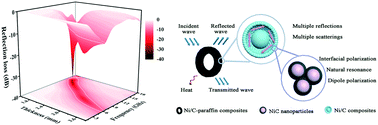
Yolk–shell Ni/C microspheres composed of Ni@C core–shell nanoparticles were successfully fabricated by decomposing a Ni-based metal–organic framework (Ni-MOF) at 500 °C and 600 °C. The Ni-MOF with a yolk–shell structure was prepared by a solvothermal method with an appropriate molar ratio of Ni(NO3)2·6H2O to C9H6O6 in the presence of PVP. The degree of crystallization of Ni and C was improved by increasing the pyrolysis temperature, which resulted in enhanced complex permittivity and optimized impedance matching of Ni/C microspheres for damping microwave. Meanwhile, the attenuation coefficient of Ni/C microspheres increased with the increment in pyrolysis temperature. The yolk–shell Ni/C microspheres obtained at 600 °C exhibited the optimal reflection loss (RL) reaching −39 dB with a bandwidth of 3.8 GHz (RL < −10 dB) at a thin matching thickness of 1.8 mm. The integrated bandwidth can achieve 12.3 GHz covering Ku-band (12–18 GHz), X-band (8–12 GHz), and most of C-band (5.7–8 GHz) with an appropriate thickness of 1.4–3.9 mm. Such excellent microwave absorption performance can be attributed to the synergistic effect of the magnetic and dielectric losses of Ni/C microspheres due to natural resonance, dipolar polarization and multiple interfacial polarizations at a unique yolk–shell interface, achieving the optimization of impedance matching and microwave attenuation. This work demonstrates that Ni/C microspheres with a desirable yolk–shell structure are potential candidates for the application in microwave absorption field.


 Please wait while we load your content...
Please wait while we load your content...