Synthesis of a novel one-dimensional Bi2O2CO3–BiOCl heterostructure and its enhanced photocatalytic activity†
Abstract
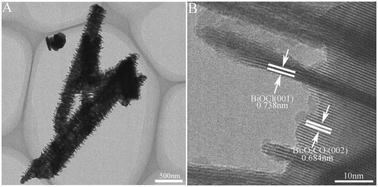
Herein, a novel one-dimensional Bi2O2CO3–BiOCl heterostructure was synthesized via a hydrothermal method. For the first time, organic solvent CH2Cl2 was used as the Cl source and reaction solvent, and a new one-dimensional Bi2O2CO3 porous rod was used as the Bi source. BiOCl nanosheets uniformly and vertically grew onto the Bi2O2CO3 porous rod by crystallographic oriented epitaxial nucleation and growth, which provides the smallest penetration barrier for photo-generated charge carrier transfer at junctions due to the formation of high-quality interfaces between Bi2O2CO3 and BiOCl. Bi2O2CO3–BiOCl displays higher photocatalytic activity than Bi2O2CO3 porous rods and BiOCl nanosheets to degrade Rhodamine B (RhB), salicylic acid (SA) and phenol. This enhanced photocatalytic activity is attributed to the synergistic effect of the following factors: the formation of a high-quality interface between Bi2O2CO3 and BiOCl, suitable band alignment of Bi2O2CO3 and BiOCl, and one-dimensional ordered nanostructure. A novel route is offered in this work to synthesize a one-dimensional Bi2O2CO3–BiOCl heterostructure with high-quality interface.


 Please wait while we load your content...
Please wait while we load your content...