A novel cadmium metal–organic framework-based multiresponsive fluorescent sensor demonstrating outstanding sensitivities and selectivities for detecting NB, Fe3+ ions and Cr2O72− anions†
Abstract
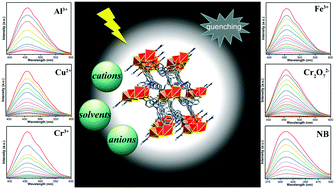
A Cd(II) metal–organic framework (MOF) [Cd3(L)(NTB)2(DMA)2]·2DMA (Cd-MOF, DMA = N,N-dimethylacetamide) was solvothermal prepared in the presence of N-donor ligand L (L = (E)-4,4′-(ethene-1,2-diyl)bis[(N-pyridin-3-yl)benzamide]) and O-donor ligand H3NTB (H3NTB = 4,4′,4′′-nitrilotribenzoic acid). The Cd-MOF is a 3D framework based on 2D [Cd3(NTB)2]n bilayers and semi-rigid bidentate L ligands, which exhibit a novel 2,3,6-connected network with {82·108·12·184}{8}6 topology. Moreover, the Cd-MOF represents the first metal–organic complex assembled by the H3NTB and bis(amide)-bis(pyridyl) co-ligands and displays remarkable fluorescence and stability as well as fluorescence quenching phenomenon towards Al3+, Cu2+, Cr3+, Fe3+ cations, Cr2O72− anion and nitrobenzene (NB). In particular, Cd-MOF can sensitively and selectively detect Fe3+, Cr2O72− and NB, and the limit of detection (LOD) values of Fe3+, Cr2O72− ions and NB are 5.1 × 10−5 M, 5.3 × 10−5 M and 4.2 × 10−5 M, respectively.


 Please wait while we load your content...
Please wait while we load your content...