A theoretical insight into non-covalent supramolecular interactions in the solid state structures of two octahedral iron(iii) complexes†
Abstract
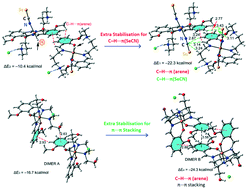
The nature and characteristics of the C–H⋯π interactions that play an important role in crystal packing of two iron(III) complexes have been discussed. [Fe(HL1)2(NCSe)2]Cl (1) and [Fe(HL2)2]Cl (2), where HL1 = 2-((3-(methylamino)propylimino)methyl)-6-ethoxyphenol and H2L2 = 2-[[[2-[(2-hydroxyethyl)amino]ethyl]imino]methyl]-6-methoxyphenol, have been synthesized and characterized by single crystal X-ray diffraction, IR, UV-visible spectroscopy and elemental analyses. Their structures have been confirmed by a single crystal X-ray diffraction study. The crystal packing of 1 shows an infinite 1D supramolecular network of C–H⋯π interactions involving either the π-cloud of the aromatic ring or the π-system of the SeCN ligand with several C–H bonds (both aliphatic and aromatic). Formation of a 2D supramolecular sheet in the solid state structure of 2 is facilitated by the C–H⋯π and π⋯π interactions. The DFT calculations have been conducted to determine the interaction energies in these complexes. The molecular electrostatic potential (MEP) surface analysis implies that the values are large and negative at the SeCN ligand (in 1) and over the aromatic ring (both in 1 and 2). Thus, both π-systems are well suited for interacting with positive MEP regions at the aliphatic and aromatic H-atoms. Moreover, the interactions have also been characterized using the non-covalent interaction (NCI) plot index computational tool.


 Please wait while we load your content...
Please wait while we load your content...