Off-stoichiometry effects on the thermoelectric properties of Cu2+δSe (−0.1 ≤ δ ≤ 0.05) compounds synthesized by a high-pressure and high-temperature method
Abstract
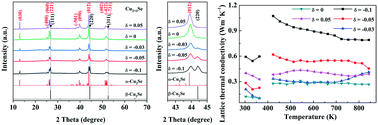
The chemical composition can directly tune the transport properties of Cu2Se liquid-like materials, including the carrier concentration, carrier mobility and superionic feature. In this work, the off-stoichiometry effects of both the deficiency and excess of Cu on the phase transition and thermoelectric properties of Cu2+δSe (−0.1 ≤ δ ≤ 0.05) compounds are investigated. The Cu2+δSe samples were synthesized by a high-pressure and high-temperature (HPHT) technique in 30 minutes. Cu2+δSe (−0.1 ≤ δ < 0) existed as a mixture of the monoclinic α-Cu2Se phase and cubic β-Cu2Se phase at room temperature, while only the monoclinic α-Cu2Se phase was detected in the Cu2+δSe (0 ≤ δ ≤ 0.05) compounds. Increasing the Cu content can effectively regulate the carrier concentration and reduce the thermal conductivity from 1.45 W m−1 K−1 (δ = −0.1) to 0.47 W m−1 K−1 (δ = 0.05) at 843 K. The ultralow lattice thermal conductivity of 0.3 W m−1 K−1 was achieved for Cu1.97Se and Cu2.0Se by stoichiometry tuning. A maximum zT value of 1.46 at 843 K was obtained for Cu2.0Se, which is 100% higher than that of Cu1.9Se. This work demonstrates the potential of off-stoichiometry for tuning the TE properties of Ag+ and Cu+ based superionic materials.


 Please wait while we load your content...
Please wait while we load your content...